Interleukin-6 overexpression induces pulmonary hypertension - PubMed (original) (raw)
Interleukin-6 overexpression induces pulmonary hypertension
M Kathryn Steiner et al. Circ Res. 2009.
Abstract
Inflammatory cytokine interleukin (IL)-6 is elevated in the serum and lungs of patients with pulmonary artery hypertension (PAH). Several animal models of PAH cite the potential role of inflammatory mediators. We investigated role of IL-6 in the pathogenesis of pulmonary vascular disease. Indices of pulmonary vascular remodeling were measured in lung-specific IL-6-overexpressing transgenic mice (Tg(+)) and compared to wild-type (Tg(-)) controls in both normoxic and chronic hypoxic conditions. The Tg(+) mice exhibited elevated right ventricular systolic pressures and right ventricular hypertrophy with corresponding pulmonary vasculopathic changes, all of which were exacerbated by chronic hypoxia. IL-6 overexpression increased muscularization of the proximal arterial tree, and hypoxia enhanced this effect. It also reproduced the muscularization and proliferative arteriopathy seen in the distal arteriolar vessels of PAH patients. The latter was characterized by the formation of occlusive neointimal angioproliferative lesions that worsened with hypoxia and were composed of endothelial cells and T-lymphocytes. IL-6-induced arteriopathic changes were accompanied by activation of proangiogenic factor, vascular endothelial growth factor, the proproliferative kinase extracellular signal-regulated kinase, proproliferative transcription factors c-MYC and MAX, and the antiapoptotic proteins survivin and Bcl-2 and downregulation of the growth inhibitor transforming growth factor-beta and proapoptotic kinases JNK and p38. These findings suggest that IL-6 promotes the development and progression of pulmonary vascular remodeling and PAH through proproliferative antiapoptotic mechanisms.
Figures
Figure 1
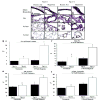
IL-6 Tg(+) mice have PAH at baseline that worsens with hypoxia. (a) RVSP is higher in Tg(+) mice (‡ vs. normoxic Tg(-);p<0.05; † vs. hypoxic Tg(-);p<0.05). (b) RV/LV+S are higher in IL-6 Tg(+) mice (‡ vs. normoxic Tg(-);p<0.05; † vs. hypoxic Tg(-);p<0.05). (c) RV weight is higher in IL-6 Tg(+) mice (‡ vs. normoxic Tg(-);p<0.05; † vs. hypoxic Tg(-);p<0.05) and increases further in hypoxia († vs. normoxic Tg(+);p<0.05) (d) Representative photomicrographs of IL-6 Tg(+) and Tg(-) mouse hearts in normoxic and hypoxic conditions. IL-6 Tg(+) mice right ventricles are hypertrophied at baseline and they hypertrophy further with hypoxia (hemotoxylin & eosin staining, magnification x25, bar=0.01mm).
Figure 2
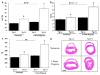
Pulmonary artery (PA) tree of IL-6 Tg(+) mice has increased muscularization that worsens with hypoxia. (a-l) Representative photomicrographs of the elastic lamina of the PA vasculature of IL-6 Tg(+) and Tg(-) mice in normoxic and hypoxic conditions. Main PA branches (Tg(-) a, b vs. Tg(+) c, d), PA at the level of the terminal bronchioles (TB) (Tg(-) e, f, vs. Tg(+) g, h), PA distal to TB (acinar) (Tg(-) i, j vs. Tg(+) k, l). Elastic tissue stain, magnification x400, bar=0.001mm. (m-p) Representative photomicrographs of smooth muscle hypertrophy of distal acinar arterioles of the PA vasculature of IL-6 Tg(+) in normoxic and hypoxic conditions (Tg(-) m, n vs. Tg(+) o, p). Immunohistochemistry with α-smooth muscle actin, magnification x400, bar=0.001mm. (q-t) Thickness of the medial wall is increased at all levels of the PA tree of IL-6 Tg(+) mice compared to Tg(-) mice at baseline and worsens with hypoxia. (q) Number of elastic lamina of main PA branches (‡ vs. normoxic Tg(-);p<0.05, † vs. normoxic Tg(+);p<0.05, and † vs. hypoxic Tg(-);p<0.05). (r) Percent wall thickness (%WT) of the main PA branches (* vs. hypoxic Tg(-);p<0.05 and * vs. normoxic Tg(+);p<0.05. ‡ vs. normoxic Tg(-);p<0.05). (s) %WT of the TB PA vessels (* vs. hypoxic Tg(-);p<0.05, * vs. normoxic Tg(+);p<0.05, † vs. normoxic Tg(-);p<0.05, ‡ vs. normoxic Tg(-);p<0.05). (t) %WT of the acinar pulmonary arteriolar vessels († vs. normoxic Tg(-);p<0.05, ‡ vs. normoxic Tg(-);p<0.05, * vs. hypoxic Tg(-);p<0.05 and * vs. normoxic Tg(+);p<0.05).
Figure 3
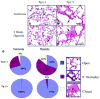
Arterioles of IL-6 Tg(+) mice have both neointimal occlusive lesions and a reduction in the total number of distal arterioles. (a-d) Representative photomicrographs of the lung parenchyma of IL-6 Tg(+) and Tg(-) mice showing neointimal hyperplasia of acinar arterioles in IL-6 Tg(+) mice in normoxic conditions (b) and occlusive arteriopathy in hypoxic conditions (d). No neointimal hyperplastic or occlusive lesions are seen in Tg(-) mice (a and c); (hematoxylin & eosin staining, magnification x400, bar=0.001mm). (e) Pie chart demonstrates that IL-6 Tg(+) mice have a higher percentage (%) of partially (P.) occluded and closed luminal acinar arterioles at baseline that worsens with hypoxia compared with Tg(-) mice. Hematoxylin & eosin stain included in key to demonstrate representative examples of an open (blue), partially occluded (red) and closed acinar arteriole (yellow), magnification x40, bar=0.001mm, arrows=arterioles.
Figure 4
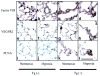
Angioproliferative lesions are present in Tg(+) mice distal arterioles. (a-l) Representative photomicrographs showing the formation of thick occlusive neointimal lesions. Endothelial cells (Factor VIII) are forming thick layers in the distal acinar arterioles of Tg(+) mice (c and d) and have increased expression of vascular endothelial growth factor receptor 2 (VEGFR2: g and h) which was not seen in Tg(-) mice (a, b or e, f). There is increased cellular proliferation in the walls of the distal arterioles of IL-6 Tg(+) mice (PCNA: k and l) in normoxic and hypoxic conditions, which was not seen in Tg(-) mice (i and j). Immunohistochemistry staining, magnification x400, bar=0.001mm.
Figure 5
Inflammatory cells present in the neointimal lesions of the arterioles of Tg(+) mice. (a-l) Representative photomicrographs showing an increase in periarteriolar lymphocytes (Giemsa stain: a-d), specifically T cells (CD3: e-h) compared to B cells (B220: i-l) in IL-6 Tg(+) mice with little change in Tg(-) mice at baseline. In hypoxic conditions, CD3+ T cells are obliterating the arteriolar lumen of IL-6 Tg(+) mice (d and h). Magnification x400, bar=0.001 mm.
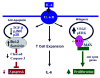
Figure 6
Hypothetical mechanisms leading to hyper-proliferative apoptotic resistant PASMC and PAEC phenotypes that result in angio-proliferative occlusive lesions and muscularization of distal vessels resulting in PAH. IL-6 induces angio-proliferative growth factor VEGF and intracellular MAPK ERK to activate pro-proliferative transcription factor complex c-MYC and MAX resulting in an increase in cell cycle genes to promote PASMC and PAEC proliferation. Concurrently, IL-6 down regulates growth inhibitor TGF-β and other MAPK that are pro-apoptotic (p38 and JNK1) while up-regulating apoptotic inhibitors survivin and Bcl-2 resulting in apoptotic resistant PASMC and PAEC phenotypes. Inflammatory cells, specifically T cells may have an important role in maintaining the secretion of the pro-proliferative cytokine IL-6.
Similar articles
- Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice.
Savale L, Tu L, Rideau D, Izziki M, Maitre B, Adnot S, Eddahibi S. Savale L, et al. Respir Res. 2009 Jan 27;10(1):6. doi: 10.1186/1465-9921-10-6. Respir Res. 2009. PMID: 19173740 Free PMC article. - Lack of bcr and abr promotes hypoxia-induced pulmonary hypertension in mice.
Yu M, Gong D, Lim M, Arutyunyan A, Groffen J, Heisterkamp N. Yu M, et al. PLoS One. 2012;7(11):e49756. doi: 10.1371/journal.pone.0049756. Epub 2012 Nov 12. PLoS One. 2012. PMID: 23152932 Free PMC article. - Deletion of LR11 Attenuates Hypoxia-Induced Pulmonary Arterial Smooth Muscle Cell Proliferation With Medial Thickening in Mice.
Jiang L, Konishi H, Nurwidya F, Satoh K, Takahashi F, Ebinuma H, Fujimura K, Takasu K, Jiang M, Shimokawa H, Bujo H, Daida H. Jiang L, et al. Arterioscler Thromb Vasc Biol. 2016 Sep;36(9):1972-9. doi: 10.1161/ATVBAHA.116.307900. Epub 2016 Aug 4. Arterioscler Thromb Vasc Biol. 2016. PMID: 27493099 - A brief overview of mouse models of pulmonary arterial hypertension: problems and prospects.
Gomez-Arroyo J, Saleem SJ, Mizuno S, Syed AA, Bogaard HJ, Abbate A, Taraseviciene-Stewart L, Sung Y, Kraskauskas D, Farkas D, Conrad DH, Nicolls MR, Voelkel NF. Gomez-Arroyo J, et al. Am J Physiol Lung Cell Mol Physiol. 2012 May 15;302(10):L977-91. doi: 10.1152/ajplung.00362.2011. Epub 2012 Feb 3. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22307907 Free PMC article. Review. - Reversible or irreversible remodeling in pulmonary arterial hypertension.
Sakao S, Tatsumi K, Voelkel NF. Sakao S, et al. Am J Respir Cell Mol Biol. 2010 Dec;43(6):629-34. doi: 10.1165/rcmb.2009-0389TR. Epub 2009 Dec 11. Am J Respir Cell Mol Biol. 2010. PMID: 20008280 Free PMC article. Review.
Cited by
- Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension.
Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, Sdrimas K, Fernandez-Gonzalez A, Kourembanas S. Lee C, et al. Circulation. 2012 Nov 27;126(22):2601-11. doi: 10.1161/CIRCULATIONAHA.112.114173. Epub 2012 Oct 31. Circulation. 2012. PMID: 23114789 Free PMC article. - Prevalence and prognostic relevance of myocardial inflammation and cardiotropic viruses in non-ischemic dilated cardiomyopathy.
Kažukauskienė I, Baltrūnienė V, Jakubauskas A, Žurauskas E, Maneikienė VV, Daunoravičius D, Čelutkienė J, Ručinskas K, Grabauskienė V. Kažukauskienė I, et al. Cardiol J. 2022;29(3):441-453. doi: 10.5603/CJ.a2020.0088. Epub 2020 Jun 22. Cardiol J. 2022. PMID: 32567670 Free PMC article. - New hope for a microRNA therapy for pulmonary arterial hypertension.
Mehta J, Parthasarathy PT, Lockey R, Kolliputi N. Mehta J, et al. Front Genet. 2013 Jul 19;4:137. doi: 10.3389/fgene.2013.00137. eCollection 2013. Front Genet. 2013. PMID: 23882281 Free PMC article. No abstract available. - Schistosomiasis and the pulmonary vasculature (2013 Grover Conference series).
Graham BB, Kumar R. Graham BB, et al. Pulm Circ. 2014 Sep;4(3):353-62. doi: 10.1086/675983. Pulm Circ. 2014. PMID: 25621148 Free PMC article. Review. - Perivascular Inflammation in Pulmonary Arterial Hypertension.
Hu Y, Chi L, Kuebler WM, Goldenberg NM. Hu Y, et al. Cells. 2020 Oct 22;9(11):2338. doi: 10.3390/cells9112338. Cells. 2020. PMID: 33105588 Free PMC article. Review.
References
- Pietra GG, Capron F, Stewart S, Leone O, Humbert M, Robbins IM, Reid LM, Tuder RM. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol. 2004;43:25S–32S. - PubMed
- Morrell NW, Yang X, Upton PD, Jourdan KB, Morgan N, Sheares KK, Trembath RC. Altered Growth Responses of Pulmonary Artery Smooth Muscle Cells From Patients With Primary Pulmonary Hypertension to Transforming Growth Factor-{beta}1 and Bone Morphogenetic Proteins. Circulation. 2001;104:790–795. - PubMed
- Blanc-Brude OP, Yu J, Simosa H, Conte MS, Sessa WC, Altieri DC. Inhibitor of apoptosis protein survivin regulates vascular injury. Nat Med. 2002;8:987–994. - PubMed
- McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, Harry G, Bonnet S, Puttagunta L, Michelakis ED. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. Journal of Clinical Investigation. 2005;115:1479–1491. [see comment] - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL039150/HL/NHLBI NIH HHS/United States
- T32 HL007874/HL/NHLBI NIH HHS/United States
- HL039150/HL/NHLBI NIH HHS/United States
- HL074859/HL/NHLBI NIH HHS/United States
- R01 HL074859/HL/NHLBI NIH HHS/United States
- T32 HL007874-11A1/HL/NHLBI NIH HHS/United States
- T32 HL007874-13/HL/NHLBI NIH HHS/United States
- HL007874/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous