Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans - PubMed (original) (raw)
Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans
Stefano Romeo et al. J Clin Invest. 2009 Jan.
Abstract
The relative activity of lipoprotein lipase (LPL) in different tissues controls the partitioning of lipoprotein-derived fatty acids between sites of fat storage (adipose tissue) and oxidation (heart and skeletal muscle). Here we used a reverse genetic strategy to test the hypothesis that 4 angiopoietin-like proteins (ANGPTL3, -4, -5, and -6) play key roles in triglyceride (TG) metabolism in humans. We re-sequenced the coding regions of the genes encoding these proteins and identified multiple rare nonsynonymous (NS) sequence variations that were associated with low plasma TG levels but not with other metabolic phenotypes. Functional studies revealed that all mutant alleles of ANGPTL3 and ANGPTL4 that were associated with low plasma TG levels interfered either with the synthesis or secretion of the protein or with the ability of the ANGPTL protein to inhibit LPL. A total of 1% of the Dallas Heart Study population and 4% of those participants with a plasma TG in the lowest quartile had a rare loss-of-function mutation in ANGPTL3, ANGPTL4, or ANGPTL5. Thus, ANGPTL3, ANGPTL4, and ANGPTL5, but not ANGPTL6, play nonredundant roles in TG metabolism, and multiple alleles at these loci cumulatively contribute to variability in plasma TG levels in humans.
Figures
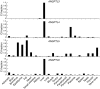
Figure 1. Expression of ANGPTL3, ANGPTL4, ANGPTL5, and ANGPTL6 in human tissues.
Quantitative real-time PCR was used to determine mRNA levels of the 4_ANGPTL_ family members in commercial cDNA arrays of 48 tissues prepared from normal humans (Origene). The 21 tissues in which the signal was detected are shown. Each bar represents the average of triplicate measurements expressed as a fraction of the Ct value obtained from the tissue expressing the highest levels of mRNA for that gene.
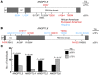
Figure 2. Schematic representation of ANGPTL3 with positions of NS sequence variations identified in the upper and lower quartiles of TG distribution in the DHS.
(A) The deduced 460–amino acid ANGPTL3 protein has the characteristic features of angiopoietins: a signal peptide (SP), an extended helical domain predicted to form coiled coils (CC), and a globular fibrinogen homology domain (FBG-like domain) at the C terminus. An excess of NS sequence variations was found in lower quartile of TG distribution compared with the upper quartile (14 vs. 5 variations; P = 0.064). Coiled coil domains were predicted using COILS (http://www.ch.embnet.org/software/COILS\_form.html), and the fibrinogen-like domain sequence was obtained from NCBI (http://www.ncbi.nlm.nih.gov/sites/entrez?db=Protein&itool=toolbar). Δ, deletion of an amino acid. (B) A common allele of_ANGPTL3_ (M259T) present in 10% of African Americans was associated with lower plasma levels of TG in 2 the DHS and the ARIC study. The variant was not associated with HDL-C or BMI. M/M, M/T, and T/T refer to predicted amino acids at position 259 of ANGPTL3.
Figure 3. Schematic representation of ANGPTL5 and ANGPTL6 with positions of NS variants identified in the upper and lower quartiles of the TG distribution in the DHS.
(A) Individuals carrying NS sequence variations in_ANGPTL5_ were more prevalent in the bottom quartile than in the top quartile (9 vs. 1 individuals, respectively; P = 0.012). (B) A corresponding analysis of ANGPTL6 revealed no significant difference in allele frequencies (6 vs. 13 individuals;P = 0.107). (C) Number of subjects with plasma TG levels in the upper quartile (n = 878) and the lower quartile (n = 897) of the DHS who had nonsense, missense, and splicing mutations in ANGPTL3, ANGPTL4,ANGPTL5, and ANGPTL6. The difference in numbers of subjects in the upper and lower quartiles is due to differences in the number of individuals with plasma TG levels at the thresholds for the quartiles. IVS, intron.
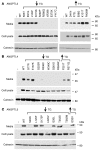
Figure 4. Effects of sequence variations on the synthesis and secretion of ANGPTL3, ANGPTL4, and ANGPTL5.
(A) ANGPTL3, (B) ANGPTL4, and (C) ANGPTL5. Wild-type and mutant forms of ANGPTL3, ANGPTL4, and ANGPTL5 were expressed in HEK293A cells, and immunoblotting was performed on the cell lysates and medium using an anti-V5 mAb as described in Methods. Calnexin was used as a loading control. This experiment was repeated 3 times with similar results. ↓TG, sequence variations found in individuals with TG in the ≤ 25th percentile; ↑TG, sequence variations found in individuals with TG in the ≥ 75th percentile; —, vector only.
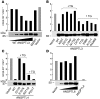
Figure 5. Effects of sequence variations in ANGPTL3 and ANGPTL4 on LPL activity.
Wild-type or mutant forms of ANGPTL3 (A and B), ANGPTL4 (C), and ANGPTL6 (D) were expressed in HEK293A cells, and conditioned medium was collected and concentrated. The effect of increasing concentrations (range of 20-fold) of protein was evaluated for wild-type ANGPTL3 (A) and ANGPTL6 (D) as described in Methods.
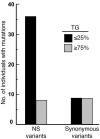
Figure 6. Cumulative frequency of NS and synonymous sequence variations in ANGPTL3, ANGPTL4, and ANGPTL5 in the upper and lower quartiles of plasma TG distribution in the DHS.
The number of individuals in each quartile was n = 878 (upper quartile) and n = 897 (lower quartile).
Similar articles
- Identification of a new functional domain in angiopoietin-like 3 (ANGPTL3) and angiopoietin-like 4 (ANGPTL4) involved in binding and inhibition of lipoprotein lipase (LPL).
Lee EC, Desai U, Gololobov G, Hong S, Feng X, Yu XC, Gay J, Wilganowski N, Gao C, Du LL, Chen J, Hu Y, Zhao S, Kirkpatrick L, Schneider M, Zambrowicz BP, Landes G, Powell DR, Sonnenburg WK. Lee EC, et al. J Biol Chem. 2009 May 15;284(20):13735-13745. doi: 10.1074/jbc.M807899200. Epub 2009 Mar 23. J Biol Chem. 2009. PMID: 19318355 Free PMC article. - A highly conserved motif within the NH2-terminal coiled-coil domain of angiopoietin-like protein 4 confers its inhibitory effects on lipoprotein lipase by disrupting the enzyme dimerization.
Yau MH, Wang Y, Lam KS, Zhang J, Wu D, Xu A. Yau MH, et al. J Biol Chem. 2009 May 1;284(18):11942-52. doi: 10.1074/jbc.M809802200. Epub 2009 Feb 26. J Biol Chem. 2009. PMID: 19246456 Free PMC article. - Regulation of triglyceride metabolism by Angiopoietin-like proteins.
Mattijssen F, Kersten S. Mattijssen F, et al. Biochim Biophys Acta. 2012 May;1821(5):782-9. doi: 10.1016/j.bbalip.2011.10.010. Epub 2011 Oct 25. Biochim Biophys Acta. 2012. PMID: 22063269 Review. - Impacts of angiopoietin-like proteins on lipoprotein metabolism and cardiovascular events.
Miida T, Hirayama S. Miida T, et al. Curr Opin Lipidol. 2010 Feb;21(1):70-5. doi: 10.1097/MOL.0b013e328333269e. Curr Opin Lipidol. 2010. PMID: 19851103 Review. - An updated ANGPTL3-4-8 model as a mechanism of triglyceride partitioning between fat and oxidative tissues.
Zhang R, Zhang K. Zhang R, et al. Prog Lipid Res. 2022 Jan;85:101140. doi: 10.1016/j.plipres.2021.101140. Epub 2021 Nov 16. Prog Lipid Res. 2022. PMID: 34793860 Free PMC article. Review.
Cited by
- Recent progress in gene therapy for familial hypercholesterolemia treatment.
Luo Y, Hou Y, Zhao W, Yang B. Luo Y, et al. iScience. 2024 Aug 10;27(9):110641. doi: 10.1016/j.isci.2024.110641. eCollection 2024 Sep 20. iScience. 2024. PMID: 39262805 Free PMC article. Review. - Low LDL-C: Is It all Good News?
Hartz J. Hartz J. Curr Atheroscler Rep. 2024 Dec;26(12):673-681. doi: 10.1007/s11883-024-01238-y. Epub 2024 Sep 10. Curr Atheroscler Rep. 2024. PMID: 39254830 Review. - Therapeutic Gene Editing in Dyslipidemias.
Tamehri Zadeh SS, Shapiro MD. Tamehri Zadeh SS, et al. Rev Cardiovasc Med. 2024 Aug 15;25(8):286. doi: 10.31083/j.rcm2508286. eCollection 2024 Aug. Rev Cardiovasc Med. 2024. PMID: 39228490 Free PMC article. Review. - Postprandial exercise regulates tissue-specific triglyceride uptake through angiopoietin-like proteins.
Liu X, Zhang Y, Han B, Li L, Li Y, Ma Y, Kang S, Li Q, Kong L, Huang K, Song BL, Liu Y, Wang Y. Liu X, et al. JCI Insight. 2024 Aug 22;9(16):e181553. doi: 10.1172/jci.insight.181553. JCI Insight. 2024. PMID: 39171527 Free PMC article. - Advances in targeting LDL cholesterol: PCSK9 inhibitors and beyond.
Safarova M, Bimal T, Soffer DE, Hirsh B, Shapiro MD, Mintz G, Cha A, Gianos E. Safarova M, et al. Am J Prev Cardiol. 2024 Jun 25;19:100701. doi: 10.1016/j.ajpc.2024.100701. eCollection 2024 Sep. Am J Prev Cardiol. 2024. PMID: 39070027 Free PMC article.
References
- Li C. Genetics and regulation of angiopoietin-like proteins 3 and 4. Curr. Opin. Lipidol. 2006;17:152–156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL020948/HL/NHLBI NIH HHS/United States
- RL1 HL092550/HL/NHLBI NIH HHS/United States
- HL-20948/HL/NHLBI NIH HHS/United States
- RL1-HL-092550/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous