Contact inhibition of locomotion in vivo controls neural crest directional migration - PubMed (original) (raw)
. 2008 Dec 18;456(7224):957-61.
doi: 10.1038/nature07441. Epub 2008 Dec 10.
Affiliations
- PMID: 19078960
- PMCID: PMC2635562
- DOI: 10.1038/nature07441
Contact inhibition of locomotion in vivo controls neural crest directional migration
Carlos Carmona-Fontaine et al. Nature. 2008.
Abstract
Contact inhibition of locomotion was discovered by Abercrombie more than 50 years ago and describes the behaviour of fibroblast cells confronting each other in vitro, where they retract their protrusions and change direction on contact. Its failure was suggested to contribute to malignant invasion. However, the molecular basis of contact inhibition of locomotion and whether it also occurs in vivo are still unknown. Here we show that neural crest cells, a highly migratory and multipotent embryonic cell population, whose behaviour has been likened to malignant invasion, demonstrate contact inhibition of locomotion both in vivo and in vitro, and that this accounts for their directional migration. When two migrating neural crest cells meet, they stop, collapse their protrusions and change direction. In contrast, when a neural crest cell meets another cell type, it fails to display contact inhibition of locomotion; instead, it invades the other tissue, in the same manner as metastatic cancer cells. We show that inhibition of non-canonical Wnt signalling abolishes both contact inhibition of locomotion and the directionality of neural crest migration. Wnt-signalling members localize at the site of cell contact, leading to activation of RhoA in this region. These results provide the first example of contact inhibition of locomotion in vivo, provide an explanation for coherent directional migration of groups of cells and establish a previously unknown role for non-canonical Wnt signalling.
Figures
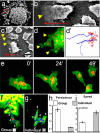
Figure 1. Cell-cell contacts polarise migrating NC cells in vitro
NC were cultured in vitro and analysed by SEM (a-c) or time-lapse microscopy of cells expressing membrane-GFP and Nuclear-RFP (d-g). Red square in a indicates leading cells, (defined by its position at the edge of migration, higher magnifications of other leading cells are shown in b and c). Arrowheads: lamellipodia (note their presence only in the leading cells, either by SEM, c; or fluorescence, d). Arrow: direction of migration. Bars in b,c: 50μm. (d') Tracks of leading (blue) and trailing (red) cells shown in d. (e) Three frames of a time-lapse for dissociated and re-aggregated NC cells. (f, g) Temporal projection to compare the migration of a group of NC cells versus individual cells. Numbers indicate the position of the same cell at different time frames 10 minutes apart. Track in a blue line. Persistence (h) and Speed (i) of migration for the migration as a group (white bar) or as an individual cell (grey bar) (p<0.005, n= 60).
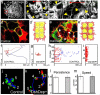
Figure 2. Contact Inhibition of Locomotion in NC cells in vitro and in vivo
(a) Experimental design. (b) Analysis of Contact Inhibition of Locomotion. Mean velocities were measured Δt minutes before and after the collision. Acceleration (red) was calculated for each cell. Angle of collision calculated after initial trajectory alignment (θ). (c-f) Invasion of confronted explants. (c) There is no invasion in NC/NC confrontations (i), outlines in (ii), overlapping area in yellow; schematised in (d). (e) NC explants completely invade and cover mesodermal explants (i), outlines in (ii), overlapping area in yellow; schematised in (f). Green arrows in f: NC path of invasion (see Supplementary Fig. 3 for supporting confocal images). (g-l) Contact Inhibition of Locomotion. (g) Collision between two pseudocoloured NC cells in vitro. Time in minutes. White arrows: direction of migration; red arrowhead: collision. (h) Velocity vectors for NC in vitro, initial velocity vector (red arrow). (i) Acceleration vectors for NC collisions in vitro. They are clustered after the collision (p<0.005, n=10). (j) Collision of two NC cells (C1, C2) in vivo shown as the difference between two consecutive two-minute frames. Green: new area; red: collapsing area; black arrow: direction of migration; red arrowhead: cell contact; white arrow: collapsing protrusion. (k) In vivo velocity vectors. (l) In vivo acceleration. They are clustered after the collision (p<0.01, n=10). (m-o) NC invasion in vivo. i, ii: lateral view; iii: transverse section along the dashed line showed in ii. NC cells are not able to invade an adjacent embryo that has NC (n; 0% of invasion, n=15), but they can to invade an embryo without NC (o; arrow, 80% of invasion, n=10). (p, q) Cell directionality in vivo. A small group of Nuclear-RFP-labelled NC cells were grafted into a normal embryo (p) or in an embryo in which the NC were previously removed (q). Note that grafted cells migrate directionally in the intact embryo (persistence: 0.6±0.04, n= 30), but not when the host NC were removed (persistence: 0.2±0.02, n=20).
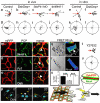
Figure 3. Effect of PCP signalling on cell contacts
(a-c). SEM of Xenopus cultured NC expressing DshDep+. Red square indicates leading cells. Higher magnification views of other leading cells are shown in b and c. Arrowheads: cell protrusions; arrow: direction of migration. Bars: 25μm in b, 50μm in c. (d, e) Two-Plane Confocal image to show cell protrusions (red) and cell shape (green). Cell protrusions are produced only at the border of the control explant (d), while they are observed between the DshDep+ cells (arrows in e). (d', e') Schematic representation of d and e. (f-i) Analysis of tracks of migrating NC cells. Blue: leading cells; red: trailing cells. Tracks of control (f) or DshDep+ (g) cells. Distribution of angles of migration for leading (blue) and trailing (red) control (h) or DshDep+ (i) cells versus the distance from the origin. (j-m) Analysis of migration of dissociated NC cells. (j, k) Five frames taken every 10 min were overlapped for a control cell (j) and a DshDep+ cell (k). Numbers: consecutive position of the cell. Blue line: track. Persistence (l) and Speed (m) were calculated for control (white bar) and DshDep+ (grey bar) cells. (p<0.05; n= 62).
Figure 4. Contact Inhibition of Locomotion: requirement of PCP and RhoA activities
Cell collisions were analysed in vivo (a-d, g-j) and in vitro (e, f, k, l). Velocities (a-f) and accelerations (g-l) were measured after the indicated treatments. Scale is the same for all panels. The change of velocity is significantly and clustered in the controls (p<0.005, n=10). No significant change is observed in any of the PCP treatments (p>>0.05, n=10 for all cases) (m-p). Different PCP components are localised at the cell-cell contact (arrowheads). mRFP: membrane-RFP; (m) Wnt11-YFP. (n) Fz7-YFP. (o) Dsh-GFP. (p) Cells expressing Dsh-GFP were analysed during a cell collision. The outline of the cell is taken from the DIC images, time in minutes; arrow: direction of migration; arrowhead: cell contact showing Dsh localization. (q-u) Role of RhoA. (q-s) FRET analysis of RhoA activity. (q) Two NC cells in contact showing RhoA activity localised at the cell contact (arrow). (r) Single NC cell. (s) RhoA FRET efficiency. Black bar: cells in contact; grey bar: single cell; white bar: single cell in which PCP has been activated by expression of DshΔN. ***: p<0.005; n= 12 each condition. (t, u) Cell collisions were analysed in the presence of the Rock inhibitor Y27632. (t) Velocity vectors; (u) acceleration vectors. No significant change in the velocity (p>>0.05, n=10) (v) Contact Inhibition of Locomotion is controlled by localization of PCP elements (red) and RhoA activity (green) at the cell contact leading to directional migration (arrows).
Similar articles
- The role of the non-canonical Wnt-planar cell polarity pathway in neural crest migration.
Mayor R, Theveneau E. Mayor R, et al. Biochem J. 2014 Jan 1;457(1):19-26. doi: 10.1042/BJ20131182. Biochem J. 2014. PMID: 24325550 Review. - Directional migration of neural crest cells in vivo is regulated by Syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA.
Matthews HK, Marchant L, Carmona-Fontaine C, Kuriyama S, Larraín J, Holt MR, Parsons M, Mayor R. Matthews HK, et al. Development. 2008 May;135(10):1771-80. doi: 10.1242/dev.017350. Epub 2008 Apr 9. Development. 2008. PMID: 18403410 - Cadherin-11 mediates contact inhibition of locomotion during Xenopus neural crest cell migration.
Becker SF, Mayor R, Kashef J. Becker SF, et al. PLoS One. 2013 Dec 31;8(12):e85717. doi: 10.1371/journal.pone.0085717. eCollection 2013. PLoS One. 2013. PMID: 24392028 Free PMC article. - Par3 controls neural crest migration by promoting microtubule catastrophe during contact inhibition of locomotion.
Moore R, Theveneau E, Pozzi S, Alexandre P, Richardson J, Merks A, Parsons M, Kashef J, Linker C, Mayor R. Moore R, et al. Development. 2013 Dec;140(23):4763-75. doi: 10.1242/dev.098509. Epub 2013 Oct 30. Development. 2013. PMID: 24173803 Free PMC article. - Signaling and transcriptional regulation in neural crest specification and migration: lessons from xenopus embryos.
Pegoraro C, Monsoro-Burq AH. Pegoraro C, et al. Wiley Interdiscip Rev Dev Biol. 2013 Mar-Apr;2(2):247-59. doi: 10.1002/wdev.76. Epub 2012 May 29. Wiley Interdiscip Rev Dev Biol. 2013. PMID: 24009035 Review.
Cited by
- ADAM13 cleavage of cadherin-11 promotes CNC migration independently of the homophilic binding site.
Abbruzzese G, Becker SF, Kashef J, Alfandari D. Abbruzzese G, et al. Dev Biol. 2016 Jul 15;415(2):383-390. doi: 10.1016/j.ydbio.2015.07.018. Epub 2015 Jul 21. Dev Biol. 2016. PMID: 26206614 Free PMC article. - Development and developmental disorders of the enteric nervous system.
Obermayr F, Hotta R, Enomoto H, Young HM. Obermayr F, et al. Nat Rev Gastroenterol Hepatol. 2013 Jan;10(1):43-57. doi: 10.1038/nrgastro.2012.234. Epub 2012 Dec 11. Nat Rev Gastroenterol Hepatol. 2013. PMID: 23229326 Review. - Chase-and-run between adjacent cell populations promotes directional collective migration.
Theveneau E, Steventon B, Scarpa E, Garcia S, Trepat X, Streit A, Mayor R. Theveneau E, et al. Nat Cell Biol. 2013 Jul;15(7):763-72. doi: 10.1038/ncb2772. Epub 2013 Jun 16. Nat Cell Biol. 2013. PMID: 23770678 Free PMC article. - Multiscale mechanisms of cell migration during development: theory and experiment.
McLennan R, Dyson L, Prather KW, Morrison JA, Baker RE, Maini PK, Kulesa PM. McLennan R, et al. Development. 2012 Aug;139(16):2935-44. doi: 10.1242/dev.081471. Epub 2012 Jul 4. Development. 2012. PMID: 22764050 Free PMC article. - Mechanisms and in vivo functions of contact inhibition of locomotion.
Stramer B, Mayor R. Stramer B, et al. Nat Rev Mol Cell Biol. 2017 Jan;18(1):43-55. doi: 10.1038/nrm.2016.118. Epub 2016 Sep 28. Nat Rev Mol Cell Biol. 2017. PMID: 27677859 Review.
References
- Abercrombie M, Heaysman JEM. Observations on the Social Behaviour of Cells in Tissue Culture .I. Speed of Movement of Chick Heart Fibroblasts in Relation to Their Mutual Contacts. Exp. Cell Res. 1953;5:111–131. - PubMed
- Abercrombie M, Heaysman JEM. Observations on the social behaviour of cells in tissue culture : II. “Monolayering” of fibroblasts. Exp. Cell Res. 1954;6:293–306. - PubMed
- Abercrombie M, Heaysman JE. Invasiveness of sarcoma cells. Nature. 1954;174:697–698. - PubMed
- Paddock SW, Dunn GA. Analysing collisions between fibroblasts and fibrosarcoma cells: fibrosarcoma cells show an active invasionary response. J. Cell Sci. 1986;81:163–187. - PubMed
- Abercrombie M. Contact Inhibition and Malignancy. Nature. 1979;281:259–262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G117/506/MRC_/Medical Research Council/United Kingdom
- G0400559/MRC_/Medical Research Council/United Kingdom
- BB/D017521/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0801145/MRC_/Medical Research Council/United Kingdom
- G0401026/MRC_/Medical Research Council/United Kingdom
- G0100152/MRC_/Medical Research Council/United Kingdom
- G0100152(56891)/MRC_/Medical Research Council/United Kingdom
- G117/506(63530)/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases