Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficiency virus-infected macaques - PubMed (original) (raw)
doi: 10.1038/mi.2008.14. Epub 2008 May 7.
C J Trindade, A Laurence, J M Heraud, J M Brenchley, M G Ferrari, L Zaffiri, E Tryniszewska, W P Tsai, M Vaccari, R Washington Parks, D Venzon, D C Douek, J J O'Shea, G Franchini
Affiliations
- PMID: 19079189
- PMCID: PMC2997489
- DOI: 10.1038/mi.2008.14
Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficiency virus-infected macaques
V Cecchinato et al. Mucosal Immunol. 2008 Jul.
Abstract
Loss of CD4(+) T cells in the gut is necessary but not sufficient to cause AIDS in animal models, raising the possibility that a differential loss of CD4(+) T-cell subtypes may be important. We found that CD4(+) T cells that produce interleukin (IL)-17, a recently identified lineage of effector CD4(+) T-helper cells, are infected by SIV(mac251)in vitro and in vivo, and are found at lower frequency at mucosal and systemic sites within a few weeks from infection. In highly viremic animals, Th1 cells predominates over Th17 T cells and the frequency of Th17 cells at mucosal sites is negatively correlated with plasma virus level. Because Th17 cells play a central role in innate and adaptive immune response to extracellular bacteria, our finding may explain the chronic enteropathy in human immunodeficiency virus (HIV) infection. Thus, therapeutic approaches that reconstitute an adequate balance between Th1 and Th17 may be beneficial in the treatment of HIV infection.
Conflict of interest statement
DISCLOSURE
The authors declared no conflict of interest.
Figures
Figure 1
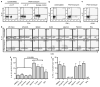
The frequency of CD3+CD4+ IL-17+ T cells is significantly higher in mucosal than systemic tissues of healthy macaques. (a) Distinct CD3+ CD4+ T-cell population produces IL-17 only after PMA/ionomycin stimulation. CD3+ CD4+ T cells were obtained from blood of a naïve macaque. Left panel: unstimulated; middle and right panels: PMA/ionomycin stimulation, cells stained with anti-IL-17 antibody and isotype control, respectively. (b) PBMCs were obtained from a naïve macaque and stimulated with or without PMA/ionomycin and costained with IL-17 and IFN-γ. Left panel: unstimulated; right panel: PMA/ionomycin stimulation. (c) Most CD3+ CD4+ IL-17+ T cells are CD25− following stimulation with PMA and ionomycin. (d) Percentage of CD4+ IL-17+ T cells in systemic and mucosal sites with or without PMA and ionomycin treatment. (e, f) Mean percentages of CD3**+** CD4+ IL-17+ T cells (e) and CD3+ CD4+ IFN-γ+ T cells (f) in tissues of healthy macaques. The number of samples analyzed for each tissue varied from four to nine. BAL, bronchial aveolar lavage; IFN, interferon; IL, interleukin; PBMC, peripheral blood mononuclear cell; PMA, phorbol 12-myristate 13-acetate.
Figure 2
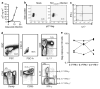
CD4+ IL-17+ T cells are susceptible to SIVmac251 infection in vitro and in vivo. (a) Viral production measured as p27 Gag in the supernatant of infected cultures by ELISA. (b) Viral production measured by intracellular staining for p27 Gag in infected and mock-infected cultures. (c) IFN-γ and IL-17 production within cells that express p27 Gag. (d) Phenotype of sorted CD4+ CD28+ CD95+ cytokine-producing cells. (e) Quantitation of SIV proviral DNA copies in sorted T-cell populations. IFN, interferon; IL, interleukin; SIV, simian immunodeficiency virus.
Figure 3
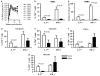
Altered balance between CD4 + IL-17+ and CD4+ IFN- γ+ T cells in primary SIVmac251 infection. (a) Plasma virus levels in macaques following intrarectal infection with SIVmac251. (b, c) Mean percentages (b) and absolute number (c) of CD4+ IL-17+ and CD4+ IFN-γ + T cells in PBMCs from naïve and infected macaques at 2 weeks from infection. The number of samples analyzed varied from four to nine. (d–g) Mean percentages of CD4 + IL-17 + and CD4 + IFN-γ + T cells in pooled lymph nodes (d), jejunum (e), colon (f), and rectum (g) from naïve and infected macaques at 2 weeks from infection. The number of samples analyzed for each tissue varied from four to nine. IFN, interferon; IL, interleukin; PBMC, peripheral blood mononuclear cell; SIV, simian immunodeficiency virus; *MamuA-01+.
Figure 4
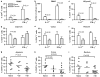
Frequency of Th17 and Th1 cells in chronically infected macaques. (a, b) Mean percentages (a) and absolute number (b) of CD4+ IL-17+ and CD4+ IFN- γ+ T cells in PBMCs from naïve and chronically SIV-infected macaques. The number of samples analyzed varied from 4 to 11. (c–f) Mean percentages of CD4+ IL-17+ and CD4+ IFN-γ+ T cells in pooled lymph nodes (c), jejunum (d), colon (e), and rectum (f) from naïve and chronically SIV-infected macaques. The number of samples analyzed for each tissue varied from 4 to 18. (g–i) Mean percentages of CD4+ IL-17+ T cells in jejunum (g), colon (h), and rectum (i) of naïve macaques and macaques with plasma virus levels below (elite controllers) or above 50 copies per ml. IFN, interferon; IL, interleukin; PBMC, peripheral blood mononuclear cell; SIV, simian immunodeficiency virus.
Figure 5
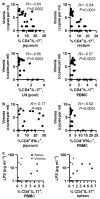
Correlative analysis of virus plasma levels and percentages of CD4+ IL-17+ T cells at mucosal sites or plasma LPS levels in infected macaques. (a) The frequency of Th17 cells in jejunum, rectum, pooled lymph nodes, and PBMCs was related to plasma virus levels (in log values) in the infected macaques. (b) The frequency of Th1 cells in jejunum and PBMCs was related to plasma virus levels (in log values) in the infected macaques. (c) The level of LPS in plasma was related to the frequency of Th17 cells in blood or spleen. No significant correlation was found in any tissue tested. IFN, interferon; IL, interleukin; LPS, lipopolysaccharide; PBMC, peripheral blood mononuclear cell.
Similar articles
- Loss of effector and anti-inflammatory natural killer T lymphocyte function in pathogenic simian immunodeficiency virus infection.
Rout N, Greene J, Yue S, O'Connor D, Johnson RP, Else JG, Exley MA, Kaur A. Rout N, et al. PLoS Pathog. 2012 Sep;8(9):e1002928. doi: 10.1371/journal.ppat.1002928. Epub 2012 Sep 20. PLoS Pathog. 2012. PMID: 23028326 Free PMC article. - Rhesus macaque resistance to mucosal simian immunodeficiency virus infection is associated with a postentry block in viral replication.
Peng B, Voltan R, Lim L, Edghill-Smith Y, Phogat S, Dimitrov DS, Arora K, Leno M, Than S, Woodward R, Markham PD, Cranage M, Robert-Guroff M. Peng B, et al. J Virol. 2002 Jun;76(12):6016-26. doi: 10.1128/jvi.76.12.6016-6026.2002. J Virol. 2002. PMID: 12021334 Free PMC article. - Suppressed Th17 levels correlate with elevated PIAS3, SHP2, and SOCS3 expression in CD4 T cells during acute simian immunodeficiency virus infection.
Bixler SL, Sandler NG, Douek DC, Mattapallil JJ. Bixler SL, et al. J Virol. 2013 Jun;87(12):7093-101. doi: 10.1128/JVI.00600-13. Epub 2013 Apr 17. J Virol. 2013. PMID: 23596301 Free PMC article. - Th17 cells in pathogenic simian immunodeficiency virus infection of macaques.
Cecchinato V, Franchini G. Cecchinato V, et al. Curr Opin HIV AIDS. 2010 Mar;5(2):141-5. doi: 10.1097/COH.0b013e32833653ec. Curr Opin HIV AIDS. 2010. PMID: 20543591 Free PMC article. Review. - Th17 cells, HIV and the gut mucosal barrier.
Dandekar S, George MD, Bäumler AJ. Dandekar S, et al. Curr Opin HIV AIDS. 2010 Mar;5(2):173-8. doi: 10.1097/COH.0b013e328335eda3. Curr Opin HIV AIDS. 2010. PMID: 20543596 Review.
Cited by
- Maintenance of intestinal Th17 cells and reduced microbial translocation in SIV-infected rhesus macaques treated with interleukin (IL)-21.
Pallikkuth S, Micci L, Ende ZS, Iriele RI, Cervasi B, Lawson B, McGary CS, Rogers KA, Else JG, Silvestri G, Easley K, Estes JD, Villinger F, Pahwa S, Paiardini M. Pallikkuth S, et al. PLoS Pathog. 2013;9(7):e1003471. doi: 10.1371/journal.ppat.1003471. Epub 2013 Jul 4. PLoS Pathog. 2013. PMID: 23853592 Free PMC article. - Mucosal immunology of HIV infection.
Xu H, Wang X, Veazey RS. Xu H, et al. Immunol Rev. 2013 Jul;254(1):10-33. doi: 10.1111/imr.12072. Immunol Rev. 2013. PMID: 23772612 Free PMC article. Review. - Th17 cells and HIV infection.
Elhed A, Unutmaz D. Elhed A, et al. Curr Opin HIV AIDS. 2010 Mar;5(2):146-50. doi: 10.1097/COH.0b013e32833647a8. Curr Opin HIV AIDS. 2010. PMID: 20543592 Free PMC article. Review. - Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections.
Estes JD, Harris LD, Klatt NR, Tabb B, Pittaluga S, Paiardini M, Barclay GR, Smedley J, Pung R, Oliveira KM, Hirsch VM, Silvestri G, Douek DC, Miller CJ, Haase AT, Lifson J, Brenchley JM. Estes JD, et al. PLoS Pathog. 2010 Aug 19;6(8):e1001052. doi: 10.1371/journal.ppat.1001052. PLoS Pathog. 2010. PMID: 20808901 Free PMC article. - Nonprogressive and progressive primate immunodeficiency lentivirus infections.
Brenchley JM, Silvestri G, Douek DC. Brenchley JM, et al. Immunity. 2010 Jun 25;32(6):737-42. doi: 10.1016/j.immuni.2010.06.004. Immunity. 2010. PMID: 20620940 Free PMC article. Review.
References
- Giorgi JV, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. - PubMed
- Hellerstein M, et al. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat Med. 1999;5:83–89. - PubMed
- Grossman Z, Meier-Schellersheim M, Paul WE, Picker LJ. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat Med. 2006;12:289–295. - PubMed
- Li Q, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. - PubMed
- Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Z01 AR041159-01/ImNIH/Intramural NIH HHS/United States
- Z01 AR041106-14/ImNIH/Intramural NIH HHS/United States
- Z01 AR041160-01/ImNIH/Intramural NIH HHS/United States
- ZIA AR041159-02/ImNIH/Intramural NIH HHS/United States
- ZIA AR041161-02/ImNIH/Intramural NIH HHS/United States
- Z01 AR041106-13/ImNIH/Intramural NIH HHS/United States
- Z01 AR041132-07/ImNIH/Intramural NIH HHS/United States
- Z01 AR041167-01/ImNIH/Intramural NIH HHS/United States
- ZIA AR041106-15/ImNIH/Intramural NIH HHS/United States
- ZIA AR041167-02/ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials