Heterogeneity of large macromolecular complexes revealed by 3D cryo-EM variance analysis - PubMed (original) (raw)
Heterogeneity of large macromolecular complexes revealed by 3D cryo-EM variance analysis
Wei Zhang et al. Structure. 2008.
Abstract
Macromolecular structure determination by cryo-electron microscopy (EM) and single-particle analysis are based on the assumption that imaged molecules have identical structure. With the increased size of processed data sets, it becomes apparent that many complexes coexist in a mixture of conformational states or contain flexible regions. We describe an implementation of the bootstrap resampling technique that yields estimates of voxel-by-voxel variance of a structure reconstructed from the set of its projections. We introduce a highly efficient reconstruction algorithm that is based on direct Fourier inversion and that incorporates correction for the transfer function of the microscope, thus extending the resolution limits of variance estimation. We also describe a validation method to determine the number of resampled volumes required to achieve stable estimate of the variance. The proposed bootstrap method was applied to a data set of 70S ribosome complexed with tRNA and the elongation factor G. The proposed method of variance estimation opens new possibilities for single-particle analysis, by extending applicability of the technique to heterogeneous data sets of macromolecules and to complexes with significant conformational variability.
Figures
Figure 1
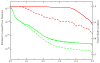
Improvement of the fidelity of the Fourier NN direct inversion 3-D reconstruction technique due to inclusion of weights accounting for uneven distribution of sampling points in Fourier space. We generated a set of evenly spaced single axis-tilt projections of a model structure (a modified 70S from E. coli placed within cube 753 voxels). To ensure that gaps existed between sampling points in Fourier space we set the angular step to 3.1°. Red lines: FSCs between original and reconstructed volume without weights (dashed) and with weights (solid). Green lines: rotationally averaged power spectra of 3D reconstructions calculated without weights (dashed), with weights (solid) and of the model structure (dashed dotted).
Figure 2
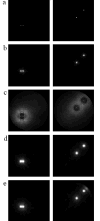
Bootstrap variance maps calculated using simulated data modified by the CTF. We show only selected z-slices, the left column is z=27, the right column is z=47. Contrast within each slice was adjusted independently, so the intensities do not reflect absolute values in respective slices. (a) Variance map of the test structures, (b) variance map obtained with CTF correction, (c) variance map obtained without CTF correction, (d) variance map obtained with CTF correction under strong low-pass filtration, (e) variance map obtained without CTF correction under strong low-pass filtration
Figure 3
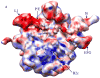
Conformational variability of the 70S•EF-G•GMPPNP complex revealed by the real-space bootstrap variance analysis of the reconstructed cryo-EM structure. The surfaces are color-coded using the values of the bootstrap standard deviation (σ) 3-D map. The red region has the highest variability (σ>0.4 g/cm3), while the blue region has the lowest variability (σ<0.1g/cm3). The panels show computationally isolated ribosomal subunits from the 3D cryo-EM reconstruction of (a) the large subunit shown from the interface side, (b) the small subunit shown from the interface side, (c) the large subunit shown from the solvent side, and (d) the small subunit shown from the solvent side. Landmarks for the subunits: L1, the L1 protuberance; P/E, the P-E-hybrid site tRNA; EFG, the Elongation Factor G; St, the L7/L12 stalk; B2c, inter-subunit bridge 2c (the center of the rotation).
Figure 3
Conformational variability of the 70S•EF-G•GMPPNP complex revealed by the real-space bootstrap variance analysis of the reconstructed cryo-EM structure. The surfaces are color-coded using the values of the bootstrap standard deviation (σ) 3-D map. The red region has the highest variability (σ>0.4 g/cm3), while the blue region has the lowest variability (σ<0.1g/cm3). The panels show computationally isolated ribosomal subunits from the 3D cryo-EM reconstruction of (a) the large subunit shown from the interface side, (b) the small subunit shown from the interface side, (c) the large subunit shown from the solvent side, and (d) the small subunit shown from the solvent side. Landmarks for the subunits: L1, the L1 protuberance; P/E, the P-E-hybrid site tRNA; EFG, the Elongation Factor G; St, the L7/L12 stalk; B2c, inter-subunit bridge 2c (the center of the rotation).
Figure 3
Conformational variability of the 70S•EF-G•GMPPNP complex revealed by the real-space bootstrap variance analysis of the reconstructed cryo-EM structure. The surfaces are color-coded using the values of the bootstrap standard deviation (σ) 3-D map. The red region has the highest variability (σ>0.4 g/cm3), while the blue region has the lowest variability (σ<0.1g/cm3). The panels show computationally isolated ribosomal subunits from the 3D cryo-EM reconstruction of (a) the large subunit shown from the interface side, (b) the small subunit shown from the interface side, (c) the large subunit shown from the solvent side, and (d) the small subunit shown from the solvent side. Landmarks for the subunits: L1, the L1 protuberance; P/E, the P-E-hybrid site tRNA; EFG, the Elongation Factor G; St, the L7/L12 stalk; B2c, inter-subunit bridge 2c (the center of the rotation).
Figure 3
Conformational variability of the 70S•EF-G•GMPPNP complex revealed by the real-space bootstrap variance analysis of the reconstructed cryo-EM structure. The surfaces are color-coded using the values of the bootstrap standard deviation (σ) 3-D map. The red region has the highest variability (σ>0.4 g/cm3), while the blue region has the lowest variability (σ<0.1g/cm3). The panels show computationally isolated ribosomal subunits from the 3D cryo-EM reconstruction of (a) the large subunit shown from the interface side, (b) the small subunit shown from the interface side, (c) the large subunit shown from the solvent side, and (d) the small subunit shown from the solvent side. Landmarks for the subunits: L1, the L1 protuberance; P/E, the P-E-hybrid site tRNA; EFG, the Elongation Factor G; St, the L7/L12 stalk; B2c, inter-subunit bridge 2c (the center of the rotation).
Similar articles
- A method of focused classification, based on the bootstrap 3D variance analysis, and its application to EF-G-dependent translocation.
Penczek PA, Frank J, Spahn CM. Penczek PA, et al. J Struct Biol. 2006 May;154(2):184-94. doi: 10.1016/j.jsb.2005.12.013. Epub 2006 Feb 17. J Struct Biol. 2006. PMID: 16520062 Review. - Dynamics of EF-G interaction with the ribosome explored by classification of a heterogeneous cryo-EM dataset.
Gao H, Valle M, Ehrenberg M, Frank J. Gao H, et al. J Struct Biol. 2004 Sep;147(3):283-90. doi: 10.1016/j.jsb.2004.02.008. J Struct Biol. 2004. PMID: 15450297 - Structure of the E. coli ribosome-EF-Tu complex at <3 Å resolution by Cs-corrected cryo-EM.
Fischer N, Neumann P, Konevega AL, Bock LV, Ficner R, Rodnina MV, Stark H. Fischer N, et al. Nature. 2015 Apr 23;520(7548):567-70. doi: 10.1038/nature14275. Epub 2015 Feb 23. Nature. 2015. PMID: 25707802 - Identifying conformational states of macromolecules by eigen-analysis of resampled cryo-EM images.
Penczek PA, Kimmel M, Spahn CM. Penczek PA, et al. Structure. 2011 Nov 9;19(11):1582-90. doi: 10.1016/j.str.2011.10.003. Structure. 2011. PMID: 22078558 Free PMC article. - Three-dimensional electron cryomicroscopy of ribosomes.
Stark H. Stark H. Curr Protein Pept Sci. 2002 Feb;3(1):79-91. doi: 10.2174/1389203023380873. Curr Protein Pept Sci. 2002. PMID: 12370013 Review.
Cited by
- Structural Study of Heterogeneous Biological Samples by Cryoelectron Microscopy and Image Processing.
White HE, Ignatiou A, Clare DK, Orlova EV. White HE, et al. Biomed Res Int. 2017;2017:1032432. doi: 10.1155/2017/1032432. Epub 2017 Jan 15. Biomed Res Int. 2017. PMID: 28191458 Free PMC article. Review. - Real-space processing of helical filaments in SPARX.
Behrmann E, Tao G, Stokes DL, Egelman EH, Raunser S, Penczek PA. Behrmann E, et al. J Struct Biol. 2012 Feb;177(2):302-13. doi: 10.1016/j.jsb.2011.12.020. Epub 2012 Jan 11. J Struct Biol. 2012. PMID: 22248449 Free PMC article. - Survey of the analysis of continuous conformational variability of biological macromolecules by electron microscopy.
Sorzano COS, Jiménez A, Mota J, Vilas JL, Maluenda D, Martínez M, Ramírez-Aportela E, Majtner T, Segura J, Sánchez-García R, Rancel Y, Del Caño L, Conesa P, Melero R, Jonic S, Vargas J, Cazals F, Freyberg Z, Krieger J, Bahar I, Marabini R, Carazo JM. Sorzano COS, et al. Acta Crystallogr F Struct Biol Commun. 2019 Jan 1;75(Pt 1):19-32. doi: 10.1107/S2053230X18015108. Epub 2019 Jan 1. Acta Crystallogr F Struct Biol Commun. 2019. PMID: 30605122 Free PMC article. Review. - CLASSIFICATION BY BOOTSTRAPPING IN SINGLE PARTICLE METHODS.
Liao HY, Frank J. Liao HY, et al. Proc IEEE Int Symp Biomed Imaging. 2010 Apr 14;2010:169-172. doi: 10.1109/ISBI.2010.5490386. Proc IEEE Int Symp Biomed Imaging. 2010. PMID: 20729994 Free PMC article. - Bayesian analysis of individual electron microscopy images: towards structures of dynamic and heterogeneous biomolecular assemblies.
Cossio P, Hummer G. Cossio P, et al. J Struct Biol. 2013 Dec;184(3):427-37. doi: 10.1016/j.jsb.2013.10.006. Epub 2013 Oct 24. J Struct Biol. 2013. PMID: 24161733 Free PMC article.
References
- Penczek P, Radermacher M, Frank J. Three-dimensional reconstruction of single particles embedded in ice. Ultramicroscopy. 1992;40:33–53. - PubMed
- Penczek PA, Grassucci RA, Frank J. The ribosome at improved resolution: new techniques for merging and orientation refinement in 3D cryo-electron microscopy of biological particles. Ultramicroscopy. 1994;53:251–270. - PubMed
- van Heel M, Gowen B, Matadeen R, Orlova EV, Finn R, Pape T, Cohen D, Stark H, Schmidt R, Schatz M, Patwardhan A. Single-particle electron cryo-microscopy: towards atomic resolution. Quarterly Reviews of Biophysics. 2000;33:307–369. - PubMed
- Harauz G, van Heel M. Exact filters for general geometry three dimensional reconstruction. Optik. 1986;73:146–156.
- Radermacher M. Weighted back-projection methods. In: Frank J, editor. Electron Tomography. New York: Plenum; 1992. pp. 91–115.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources