Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances - PubMed (original) (raw)
Review
Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances
Joseph J Volpe. Lancet Neurol. 2009 Jan.
Abstract
Brain injury in premature infants is of enormous public health importance because of the large number of such infants who survive with serious neurodevelopmental disability, including major cognitive deficits and motor disability. This type of brain injury is generally thought to consist primarily of periventricular leukomalacia (PVL), a distinctive form of cerebral white matter injury. Important new work shows that PVL is frequently accompanied by neuronal/axonal disease, affecting the cerebral white matter, thalamus, basal ganglia, cerebral cortex, brain stem, and cerebellum. This constellation of PVL and neuronal/axonal disease is sufficiently distinctive to be termed "encephalopathy of prematurity". The thesis of this Review is that the encephalopathy of prematurity is a complex amalgam of primary destructive disease and secondary maturational and trophic disturbances. This Review integrates the fascinating confluence of new insights into both brain injury and brain development during the human premature period.
Conflict of interest statement
Conflicts of interest
I have no conflicts of interest.
Figures
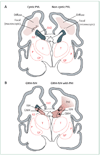
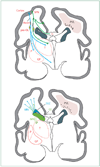
Figure 1. Cystic and non-cystic periventricular leukomalacia (PVL) and germinal matrix haemorrhage–intraventricular haemorrhage (GMH-IVH) and GMH-IVH with periventricular haemorrhagic infarction (PHI)
Coronal sections from the brain of a 28-week-old premature infant. The dorsal cerebral subventricular zone (SVZ), the ventral germinative epithelium of the ganglionic eminence (GE), thalamus (T), and putamen (P)/globus pallidus (GP) are shown. (A) The focal necrotic lesions in cystic PVL (small circles) are macroscopic in size and evolve to cysts. The focal necrotic lesions in non-cystic PVL (black dots) are microscopic in size and evolve to glial scars. The diffuse component of both cystic and non-cystic PVL (pink) is characterised by the cellular changes, as described in the text. (B) Haemorrhage (red) into the GE results in GMH, which could burst through the ependyma to cause an IVH (left). When the GHM-IVH is large, PHI might result (right).
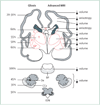
Figure 2. Main neuronal/axonal structures affected in premature infants with periventricular leukomalacia
Coronal sections of the cerebrum, pons, cerebellum, and medulla (inferior olivary nuclei) are shown. The frequency of gliosis by neuropathological study and the major abnormalities detected by advanced MRI (volumetric and diffusion-based MRI) are shown. See text for details. BP=basis pontis. C=caudate. CC=corpus callosum. CCx=cerebellar cortex. De=dentate. GP=globus pallidus. ION=inferior olivary nuclei. P=putamen. T=thalamus.
Figure 3. Cerebrum in coronal section at 28 weeks’ gestation showing critical events in cortical development
(A) The axons (green) emanate from the thalamus (T; projection fibres), corpus callosum (CC; commissural fibres), and cortex (association fibres), which synapse initially on subplate neurons (SPNs). SPNs send axons to the cortex and promote cortical development before the thalamo-cortical and cortico-cortical fibres enter the cortex. From the cortex, axons (blue) descend to the thalamus, basal ganglia, and corticospinal (and corticopontine) tracts. Premyelinating oligodendrocytes (pre-OLs; yellow) enstheath axons before full differentiation to mature myelin-producing oligodendrocytes. (B) The proliferation and migration of GABAergic interneurons from the subventricular zone (SVZ) and ventral germinative epithelium of the ganglionic eminence (GE) are shown. Neurons from the SVZ (blue) migrate radially to the cortex and from the GE (green), tangentially and then radially, to the cortex. The migrating stream of interneurons from the GE to the dorsal thalamus is also shown. GP=globus pallidus. MN=migrating neurons. P=putamen.
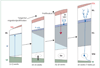
Figure 4. The developing cerebellar cortex at four major time periods from 9 weeks’ gestation to 7 weeks into the postnatal period
The two proliferative zones are the ventricular zone (VZ) and the external granule cell layer (EGL) derived from the rhombic lip. The VZ gives rise to the Purkinje cells and the deep nuclei (dentate nucleus [De]). The granule precursor cells of the EGL migrate over the surface of the cerebellum. Proliferation in the EGL is activated by sonic hedgehog homologue (SHH), secreted by Purkinje cells (P-cells). The proliferating cells are concentrated in the outer half of the EGL. When post-mitotic, these cells migrate radially inwards (guided by Bergman glia [not shown]) to form the internal granule cell layer (IGL). Note the markedly active proliferation and migration of the granule precursor cells of the EGL during the premature period. ep=ependyma. ML=molecular layer. IZ=intermediate zone. pn=postnatal. WM=white matter. Adapted from ten-Donkelaar et al, with permission from Springer.
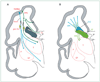
Figure 5. Potential sequences of events leading to major brain sequelae observed with periventricular leukomalacia
Potential events are hypomyelination, and impaired cortical and thalamic development (eg, seen on advanced MRI analysis by decreased volume). For each sequence, the initiating primary injury is shown, and the subsequent secondary effects are postulated to occur because of maturational/trophic disturbances, as described in the text. Pre-OL=premyelinating oligodendrocyte. SPN=subplate neuron.
Figure 6. Anatomical relationships between the major developmental events and the topography of non-cystic periventricular leukomalacia (PVL)
For purposes of clarity, the developmental events are separated. CC=corpus callosum. GE=ganglionic eminence. GP=globus pallidus. MN=migrating neurons. P=putamen. Pre-OL=premyelinating oligodendrocytes. SPN=subplate neurons. SVZ=subventricular zone. T=thalamus.
Figure 7. Likely sequence of events leading to the major neuropathological sequelae observed with germinal matrix haemorrhage (GMH)–intraventricular haemorrhage (IVH) with periventricular haemorrhagic infarction (PHI)
The primary destructive events involve the GMH-IVH with PHI and the associated destruction of premyelinating oligodendrocytes (pre-OLs) and axons. The secondary consequence of the former is hypomyelination, and that of the latter is impaired thalamic and cortical development. In addition, because of destruction of the dorsal subventricular zone (SVZ) and ventral germinative epithelium of the ganglionic eminence (GE), impaired proliferation and late migration of GABAergic interneurons to upper cortical layers and the thalamus could contribute to defective cortical and thalamic development.
Figure 8. The anatomical relationships between the major developmental events and the topography of germinal matrix haemorrhage (GMH)–intraventricular haemorrhage (IVH) with periventricular haemorrhagic infarction (PHI)
For purposes of clarity, the developmental events are separated. CC=corpus callosum. GE=ganglionic eminence. GP=globus pallidus. MN=migrating neurons. P=putamen. Pre-OL=premyelinating oligodendrocytes. SPN=subplate neurons. SVZ=subventricular zone. T=thalamus.
Similar articles
- Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term.
Inder TE, Huppi PS, Warfield S, Kikinis R, Zientara GP, Barnes PD, Jolesz F, Volpe JJ. Inder TE, et al. Ann Neurol. 1999 Nov;46(5):755-60. doi: 10.1002/1531-8249(199911)46:5<755::aid-ana11>3.0.co;2-0. Ann Neurol. 1999. PMID: 10553993 - Gray matter injury associated with periventricular leukomalacia in the premature infant.
Pierson CR, Folkerth RD, Billiards SS, Trachtenberg FL, Drinkwater ME, Volpe JJ, Kinney HC. Pierson CR, et al. Acta Neuropathol. 2007 Dec;114(6):619-31. doi: 10.1007/s00401-007-0295-5. Epub 2007 Oct 3. Acta Neuropathol. 2007. PMID: 17912538 Free PMC article. - The encephalopathy of prematurity--brain injury and impaired brain development inextricably intertwined.
Volpe JJ. Volpe JJ. Semin Pediatr Neurol. 2009 Dec;16(4):167-78. doi: 10.1016/j.spen.2009.09.005. Semin Pediatr Neurol. 2009. PMID: 19945651 Free PMC article. Review. - Emerging concepts in periventricular white matter injury.
Back SA, Rivkees SA. Back SA, et al. Semin Perinatol. 2004 Dec;28(6):405-14. doi: 10.1053/j.semperi.2004.10.010. Semin Perinatol. 2004. PMID: 15693397 Review. - Diffuse axonal injury in periventricular leukomalacia as determined by apoptotic marker fractin.
Haynes RL, Billiards SS, Borenstein NS, Volpe JJ, Kinney HC. Haynes RL, et al. Pediatr Res. 2008 Jun;63(6):656-61. doi: 10.1203/PDR.0b013e31816c825c. Pediatr Res. 2008. PMID: 18520330 Free PMC article.
Cited by
- Subcortical Change and Neurohabilitation Treatment Adherence Effects in Extremely Preterm Children.
Castro-Chavira SA, Gutiérrez-Hernández CC, Carrillo-Prado C, Harmony T. Castro-Chavira SA, et al. Brain Sci. 2024 Sep 25;14(10):957. doi: 10.3390/brainsci14100957. Brain Sci. 2024. PMID: 39451972 Free PMC article. - Association of Infection in Neonates and Long-Term Neurodevelopmental Outcome.
Sewell E, Roberts J, Mukhopadhyay S. Sewell E, et al. Clin Perinatol. 2021 Jun;48(2):251-261. doi: 10.1016/j.clp.2021.03.001. Clin Perinatol. 2021. PMID: 34030812 Free PMC article. Review. - Intranasal IL-4 Administration Alleviates Functional Deficits of Periventricular Leukomalacia in Neonatal Mice.
Yu LC, Miao JK, Li WB, Chen N, Chen QX. Yu LC, et al. Front Neurol. 2020 Sep 2;11:930. doi: 10.3389/fneur.2020.00930. eCollection 2020. Front Neurol. 2020. PMID: 32982939 Free PMC article. - Systemic multipotent adult progenitor cells protect the cerebellum after asphyxia in fetal sheep.
Gussenhoven R, Ophelders DRMG, Dudink J, Pieterman K, Lammens M, Mays RW, Zimmermann LJ, Kramer BW, Wolfs TGAM, Jellema RK. Gussenhoven R, et al. Stem Cells Transl Med. 2021 Jan;10(1):57-67. doi: 10.1002/sctm.19-0157. Epub 2020 Sep 28. Stem Cells Transl Med. 2021. PMID: 32985793 Free PMC article. - Case Series: Fractional Anisotropy Profiles of the Cerebellar Peduncles in Adolescents Born Preterm With Ventricular Dilation.
Travis KE, Leitner Y, Ben-Shachar M, Yeom KW, Feldman HM. Travis KE, et al. J Child Neurol. 2016 Mar;31(3):321-7. doi: 10.1177/0883073815592223. Epub 2015 Jun 26. J Child Neurol. 2016. PMID: 26116381 Free PMC article.
References
- Martin JA, Kung HC, Mathews TJ, et al. Annual summary of vital statistics: 2006. Pediatrics. 2008;121:788–801. - PubMed
- Woodward LJ, Edgin JO, Thompson D, Inder TE. Object working memory deficits predicted by early brain injury and development in the preterm infant. Brain. 2005;128:2578–2587. - PubMed
- Bayless S, Stevenson J. Executive functions in school-age children born very prematurely. Early Hum Dev. 2007;83:247–254. - PubMed
- Platt MJ, Cans C, Johnson A, et al. Trends in cerebral palsy among infants of very low birthweight (<1500 g) or born prematurely (<32 weeks) in 16 European centres: a database study. Lancet. 2007;369:43–50. - PubMed
- Larroque B, Ancel PY, Marret S, et al. Neurodevelopmental disabilities and special care of 5-year-old children born before 33 weeks of gestation (the EPIPAGE study): a longitudinal cohort study. Lancet. 2008;371:813–820. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical