Real-time visualization of dynamin-catalyzed membrane fission and vesicle release - PubMed (original) (raw)
Real-time visualization of dynamin-catalyzed membrane fission and vesicle release
Thomas J Pucadyil et al. Cell. 2008.
Abstract
The GTPase dynamin assembles at the necks of budded vesicles in vivo and functions in membrane fission. We have developed fluid supported bilayers with excess membrane reservoir, (SUPER) templates, to assay vesicle formation and membrane fission. Consistent with previous studies, in the absence of GTP, dynamin assembles in spirals, forming long membrane tubules. GTP addition triggers disassembly, but not membrane fission, arguing against models in which fission is mediated by concerted and global GTP-driven conformational changes. In contrast, under physiological conditions in the constant presence of GTP, dynamin mediates membrane fission. Under these conditions, fluorescently labeled dynamin cooperatively organizes into self-limited assemblies that continuously cycle at the membrane and drive vesicle release. When visualized at the necks of emergent vesicles, self-limited dynamin assemblies display intensity fluctuations and persist for variable time periods before fission. Thus, self-limited assemblies of dynamin generated in the constant presence of GTP catalyze membrane fission.
Figures
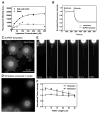
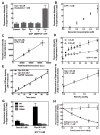
Figure 1. Supported bilayers with excess membrane reservoir (SUPER templates)
(A) Fluorescence intensity on silica beads incubated with increasing concentrations of DOPC:DOPS:PI-4,5-P2:RhPE (79:15:5:1 mol%) liposomes in either 600 mM salt buffer or water and subsequently washed with water. Data represent the means±SD (n=3). (B) Dithionite-mediated quenching of fluorescence in DOPC:DOPS:PI-4,5-P2:NBD-PE (78:15:5:2 mol%) liposomes and SUPER templates formed from these liposomes. Plots are the mean of n=5 independent fluorescence intensity traces. Fluorescence images of (C) SUPER templates (see Movie S1) and (D) templates prepared in water added to a glass coverslip. (E) Extension and retraction of a membrane tether from a SUPER template (see Movie S2). (F) Fluorescence intensities in the ROI in (E, white square) plotted as a function of tether length. Data in extension plots are normalized to the tether intensity at 5 μm and in retraction plots to tether intensity at 30 μm. Data represent the mean±SD (n=5).
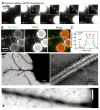
Figure 2. Dynamin-induced membrane tubulation from SUPER templates
(A) Time-lapse images showing the effect of adding dynamin to templates. See Movie S3. Insets are relevant portions in respective frames adjusted for contrast. (B) Fluorescence of BODIPY-dynamin (Bod-Dyn) and membrane (RhPE) on membrane tubules and SUPER templates. (C) Fluorescence intensity across the dotted line shown in (B). Negative-stain EM of a dynamin-coated coiled membrane tubule at low (D) and high (E) magnification. (F) Negative-stain EM showing the dynamin scaffold on an individual membrane tubule.
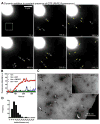
Figure 3. Dynamin-catalyzed membrane fission leading to vesicle release
(A) Time-lapse images showing the effect of adding dynamin to SUPER templates in the presence of GTP (see Movie S4). (B) Mean fluorescence intensities in a small area of the bathing solution (white square in A) monitored after dynamin addition to templates in buffer alone or with GMPPCP, GDP, or GTP. Black arrow marks dynamin addition. (C) Negative-stain EM of the bathing solution after adding dynamin and GTP to templates. Inset shows a negative-stain EM of the bathing solution after adding dynamin to templates in the absence of GTP. (D) Size distribution of vesicles (n=221) measured from EM micrographs.
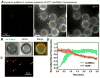
Figure 4. Dynamin behavior during membrane fission
(A) Time-lapse images showing the effect of adding Ax488dyn to templates in presence of GTP (see Movie S5). (B) Colocalization of punctate Ax488dyn fluorescence on the template with high RhPE fluorescence in presence of GTP. Ax488dyn and RhPE images are inverted in contrast for clarity. (C) Colocalization of Ax488dyn and RhPE fluorescence in the bathing solution in the presence of GTP. (D) Plots showing background corrected Ax488dyn and RhPE fluorescence on templates in the presence of GTP. Data represents the mean±SD (n=8).
Figure 5. Biochemical analysis of membrane fission by sedimentation assays
(A) Nucleotide dependence for dynamin-catalyzed membrane fission. (B) Dependence of membrane fission on dynamin concentration. Data are corrected for background fluorescence in the supernatant of control samples. (C) Dependence of membrane fission on concentration of SUPER templates. Data are corrected for background fluorescence in the supernatant of control samples. (D) Dependence of fission kinetics on dynamin concentration. (E) Dynamin GTPase activity in the presence and absence of SUPER templates. (F) Dependence of fission on PI-4,5-P2 concentration in the membrane. Data are normalized to fluorescence in supernatant of samples with dynamin alone. (G) Comparison of membrane fission with wild type (WT), GTP-binding defective (S45N), and self-assembly defective (R399A) dynamin. (H) Effect of increasing concentrations of S45N (filled circles) and R399A (open circles) on WT dynamin-catalyzed membrane fission. In all cases, dynamin and nucleotides were premixed in solution before addition of templates. All data represent the mean±SD (n≥3).
Figure 6. Behavior of preassembled dynamin in response to GTP addition
(A) Time-lapse images showing the effect of GTP addition to an apparent network of bundled membrane tubules of preassembled dynamin (see Movie S8). (B) Plots showing background corrected Ax488dyn and RhPE fluorescence on templates before and after GTP addition. Data represent the mean±SD (n=8). (C) Time-lapse images showing the effect of GTP addition to dynamin-coated bundled membrane tubules tethered between templates (see Movie S9). (D) Time-lapse images showing the effect of GTP addition to preassembled BODIPY-dynamin on bundled membrane tubules tethered between templates (see Movie S10). (E) Dynamin-catalyzed membrane fission after dynamin preassembly. Dynamin was preassembled on SUPER templates for 10 min before GTP addition. Data are corrected for background fluorescence in supernatant from control samples. (F) Dependence of extent of fission on the duration for which dynamin is preassembled on the template. All data represent the mean±SD (n≥3).
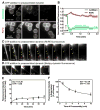
Figure 7. Visualizing dynamin-catalyzed membrane fission
(A) Time-lapse images showing the effect of adding dynamin suspended in glycerol (final dynamin concentration=0.5 μM, final glycerol concentration=2%) to SUPER templates. Insets are relevant portions in respective frames adjusted for contrast. See Movie S12. (B) Size distribution of buds (n=77) measured from fluorescence micrographs. (C) Time-lapse images showing the effect of adding dynamin suspended in glycerol to templates in the presence of GTP (see Movie S13). (D) Time-lapse images showing the effect of dynamin on a membrane template of conjoined buds in the presence of GTP (see Movie S14). (E) Time-lapse images showing the effect of adding BODIPY-dynamin suspended in glycerol to templates in the presence of GTP. Insets in the first two frames are relevant portions in respective frames adjusted for contrast. See Movie S15. (F) Plots showing fluorescence of BODIPY-dynamin at the neck (red trace) and the bud (blue trace) during a fission event of the large bud shown in (E). (G) Time-lapse images showing the effect of adding dynamin to a membrane template of partially retracted membrane tethers generated from SUPER templates in the presence of GTP (see Movie S16).
Comment in
- The long and short of membrane fission.
Roux A, Antonny B. Roux A, et al. Cell. 2008 Dec 26;135(7):1163-5. doi: 10.1016/j.cell.2008.12.003. Cell. 2008. PMID: 19109885
Similar articles
- GTPase cycle of dynamin is coupled to membrane squeeze and release, leading to spontaneous fission.
Bashkirov PV, Akimov SA, Evseev AI, Schmid SL, Zimmerberg J, Frolov VA. Bashkirov PV, et al. Cell. 2008 Dec 26;135(7):1276-86. doi: 10.1016/j.cell.2008.11.028. Epub 2008 Dec 11. Cell. 2008. PMID: 19084269 Free PMC article. - Dynamin: functional design of a membrane fission catalyst.
Schmid SL, Frolov VA. Schmid SL, et al. Annu Rev Cell Dev Biol. 2011;27:79-105. doi: 10.1146/annurev-cellbio-100109-104016. Epub 2011 May 18. Annu Rev Cell Dev Biol. 2011. PMID: 21599493 Review. - Cryo-EM structures of membrane-bound dynamin in a post-hydrolysis state primed for membrane fission.
Jimah JR, Kundu N, Stanton AE, Sochacki KA, Canagarajah B, Chan L, Strub MP, Wang H, Taraska JW, Hinshaw JE. Jimah JR, et al. Dev Cell. 2024 Jul 22;59(14):1783-1793.e5. doi: 10.1016/j.devcel.2024.04.008. Epub 2024 Apr 24. Dev Cell. 2024. PMID: 38663399 - Dynamin-catalyzed membrane fission requires coordinated GTP hydrolysis.
Liu YW, Mattila JP, Schmid SL. Liu YW, et al. PLoS One. 2013;8(1):e55691. doi: 10.1371/journal.pone.0055691. Epub 2013 Jan 31. PLoS One. 2013. PMID: 23383266 Free PMC article. - Building a fission machine--structural insights into dynamin assembly and activation.
Chappie JS, Dyda F. Chappie JS, et al. J Cell Sci. 2013 Jul 1;126(Pt 13):2773-84. doi: 10.1242/jcs.108845. Epub 2013 Jun 18. J Cell Sci. 2013. PMID: 23781021 Free PMC article. Review.
Cited by
- Geometric catalysis of membrane fission driven by flexible dynamin rings.
Shnyrova AV, Bashkirov PV, Akimov SA, Pucadyil TJ, Zimmerberg J, Schmid SL, Frolov VA. Shnyrova AV, et al. Science. 2013 Mar 22;339(6126):1433-6. doi: 10.1126/science.1233920. Science. 2013. PMID: 23520112 Free PMC article. - A neurotoxic phospholipase A2 impairs yeast amphiphysin activity and reduces endocytosis.
Mattiazzi M, Sun Y, Wolinski H, Bavdek A, Petan T, Anderluh G, Kohlwein SD, Drubin DG, Križaj I, Petrovič U. Mattiazzi M, et al. PLoS One. 2012;7(7):e40931. doi: 10.1371/journal.pone.0040931. Epub 2012 Jul 23. PLoS One. 2012. PMID: 22844417 Free PMC article. - Dual role of BAR domain-containing proteins in regulating vesicle release catalyzed by the GTPase, dynamin-2.
Neumann S, Schmid SL. Neumann S, et al. J Biol Chem. 2013 Aug 30;288(35):25119-25128. doi: 10.1074/jbc.M113.490474. Epub 2013 Jul 16. J Biol Chem. 2013. PMID: 23861397 Free PMC article. - Membrane fusion by Drosophila atlastin does not require GTP hydrolysis.
Crosby D, Lee TH. Crosby D, et al. Mol Biol Cell. 2022 Dec 1;33(14):br23. doi: 10.1091/mbc.E22-05-0164. Epub 2022 Sep 21. Mol Biol Cell. 2022. PMID: 36129776 Free PMC article. - Membrane curvature controls dynamin polymerization.
Roux A, Koster G, Lenz M, Sorre B, Manneville JB, Nassoy P, Bassereau P. Roux A, et al. Proc Natl Acad Sci U S A. 2010 Mar 2;107(9):4141-6. doi: 10.1073/pnas.0913734107. Epub 2010 Feb 16. Proc Natl Acad Sci U S A. 2010. PMID: 20160074 Free PMC article.
References
- Antonny B. Membrane deformation by protein coats. Curr Opin Cell Biol. 2006;18:386–394. - PubMed
- Baksh MM, Jaros M, Groves JT. Detection of molecular interactions at membrane surfaces through colloid phase transitions. Nature. 2004;427:139–141. - PubMed
- Bigay J, Gounon P, Robineau S, Antonny B. Lipid packing sensed by ArfGAP1 couples COPI coat disassembly to membrane bilayer curvature. Nature. 2003;426:563–566. - PubMed
- Chernomordik LV, Kozlov MM. Protein-lipid interplay in fusion and fission of biological membranes. Annu Rev Biochem. 2003;72:175–207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 MH061345/MH/NIMH NIH HHS/United States
- R01 MH061345/MH/NIMH NIH HHS/United States
- R37MH61345/MH/NIMH NIH HHS/United States
- R01 GM042455/GM/NIGMS NIH HHS/United States
- R01GM042455/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources