Small G proteins as key regulators of pancreatic digestive enzyme secretion - PubMed (original) (raw)
Review
Small G proteins as key regulators of pancreatic digestive enzyme secretion
John A Williams et al. Am J Physiol Endocrinol Metab. 2009 Mar.
Abstract
Small GTP-binding (G) proteins act as molecular switches to regulate a number of cellular processes, including vesicular transport. Emerging evidence indicates that small G proteins regulate a number of steps in the secretion of pancreatic acinar cells. Diverse small G proteins have been localized at discrete compartments along the secretory pathway and particularly on the secretory granule. Rab3D, Rab27B, and Rap1 are present on the granule membrane and play a role in the steps leading up to exocytosis. Whether the function of these G proteins is simply to ensure appropriate targeting or if they are involved as regulatory molecules is discussed. Most evidence suggests that Rab3D and Rab27B play a role in tethering the secretory granule to its target membrane. Other Rabs have been identified on the secretory granule that are associated with different steps in the secretory pathway. The Rho family small G proteins RhoA and Rac1 also regulate secretion through remodeling of the actin cytoskeleton. Possible mechanisms for regulation of these G proteins and their effector molecules are considered.
Figures
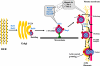
Fig. 1.
Small G proteins as regulators of the steps by which secretory proteins move through the secretory pathway from rough endoplasmic reticulum (RER) to enzyme release by exocytosis. TGN, trans-Golgi network. Small G proteins are listed in blue and shown next to steps where they have been identified. Other proteins identified on zymogen granules (ZGs), which may play a role in secretion, are listed in black. R_ight_: mature ZGs pass through the actin filaments of the terminal web with the aid of RhoA and Rac1, are attached to the membrane by tethering proteins, and then dock and fuse with the apical membrane to release their contents. These terminal steps involve the participation of SNARE proteins. At the time of fusion, the granules also become coated with actin, and at least some of the G proteins are released.
Similar articles
- Genetic deletion of Rab27B in pancreatic acinar cells affects granules size and has inhibitory effects on amylase secretion.
Hou Y, Ernst SA, Lentz SI, Williams JA. Hou Y, et al. Biochem Biophys Res Commun. 2016 Mar 18;471(4):610-5. doi: 10.1016/j.bbrc.2016.01.180. Epub 2016 Feb 2. Biochem Biophys Res Commun. 2016. PMID: 26845357 Free PMC article. - Functional role of J domain of cysteine string protein in Ca2+-dependent secretion from acinar cells.
Weng N, Baumler MD, Thomas DD, Falkowski MA, Swayne LA, Braun JE, Groblewski GE. Weng N, et al. Am J Physiol Gastrointest Liver Physiol. 2009 May;296(5):G1030-9. doi: 10.1152/ajpgi.90592.2008. Epub 2009 Mar 12. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19282376 Free PMC article. - Rab8 is involved in zymogen granule formation in pancreatic acinar AR42J cells.
Faust F, Gomez-Lazaro M, Borta H, Agricola B, Schrader M. Faust F, et al. Traffic. 2008 Jun;9(6):964-79. doi: 10.1111/j.1600-0854.2008.00739.x. Epub 2008 Mar 24. Traffic. 2008. PMID: 18363906 - Cell secretion: current structural and biochemical insights.
Trikha S, Lee EC, Jeremic AM. Trikha S, et al. ScientificWorldJournal. 2010 Oct 12;10:2054-69. doi: 10.1100/tsw.2010.193. ScientificWorldJournal. 2010. PMID: 20953555 Free PMC article. Review. - [Small, monomeric G proteins in plants].
Kowalczyk S, Hetmann A, Czarnota M. Kowalczyk S, et al. Postepy Biochem. 2011;57(4):442-53. Postepy Biochem. 2011. PMID: 22568176 Review. Polish.
Cited by
- Morphological and histochemical characterization of the secretory epithelium in the canine lacrimal gland.
Yasui T, Miyata K, Nakatsuka C, Tsukise A, Gomi H. Yasui T, et al. Eur J Histochem. 2021 Nov 2;65(4):3320. doi: 10.4081/ejh.2021.3320. Eur J Histochem. 2021. PMID: 34726360 Free PMC article. - Directing exocrine secretory vesicles to the apical membrane by actin cables generated by the formin mDia1.
Geron E, Schejter ED, Shilo BZ. Geron E, et al. Proc Natl Acad Sci U S A. 2013 Jun 25;110(26):10652-7. doi: 10.1073/pnas.1303796110. Epub 2013 Jun 10. Proc Natl Acad Sci U S A. 2013. PMID: 23754409 Free PMC article. - Regulation of acinar cell function in the pancreas.
Williams JA. Williams JA. Curr Opin Gastroenterol. 2010 Sep;26(5):478-83. doi: 10.1097/MOG.0b013e32833d11c6. Curr Opin Gastroenterol. 2010. PMID: 20625287 Free PMC article. Review. - Rap1-Rac1 circuits potentiate platelet activation.
Stefanini L, Boulaftali Y, Ouellette TD, Holinstat M, Désiré L, Leblond B, Andre P, Conley PB, Bergmeier W. Stefanini L, et al. Arterioscler Thromb Vasc Biol. 2012 Feb;32(2):434-41. doi: 10.1161/ATVBAHA.111.239194. Epub 2011 Nov 10. Arterioscler Thromb Vasc Biol. 2012. PMID: 22075250 Free PMC article. - MIST1 and PTF1 Collaborate in Feed-Forward Regulatory Loops That Maintain the Pancreatic Acinar Phenotype in Adult Mice.
Jiang M, Azevedo-Pouly AC, Deering TG, Hoang CQ, DiRenzo D, Hess DA, Konieczny SF, Swift GH, MacDonald RJ. Jiang M, et al. Mol Cell Biol. 2016 Nov 14;36(23):2945-2955. doi: 10.1128/MCB.00370-16. Print 2016 Dec 1. Mol Cell Biol. 2016. PMID: 27644326 Free PMC article.
References
- Aktories K, Braun U, Rosener S, Just I, Hall A. The rho gene product expressed in E. coli is a substrate of botulinum ADP-ribosyltransferase C3. Biochem Biophys Res Commun 158: 209–213, 1989. - PubMed
- Amano R, Lee J, Goto N, Harayama H. Evidence for existence of cAMP-Epac signaling in the heads of mouse epididymal spermatozoa. J Reprod Dev 53: 127–133, 2007. - PubMed
- Bi Y, Page SL, Williams JA. Rho and Rac promote acinar morphological changes, actin reorganization, and amylase secretion. Am J Physiol Gastrointest Liver Physiol 289: G561–G570, 2005. - PubMed
- Bi Y, Williams JA. A role for Rho and Rac in secretagogue-induced amylase release by pancreatic acini. Am J Physiol Cell Physiol 289: C22–C32, 2005. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials