Murine cytomegalovirus displays selective infection of cells within hours after systemic administration - PubMed (original) (raw)
Murine cytomegalovirus displays selective infection of cells within hours after systemic administration
Kimberly M Hsu et al. J Gen Virol. 2009 Jan.
Abstract
A distinctive feature of the cytomegaloviruses is their wide tissue tropism, demonstrated by the infection of many organs and cell types in an active infection. However, in experimental models of systemic infection, the earliest stages of infection are not well characterized, and it is unclear whether only certain cells are initially infected. Using a recombinant murine cytomegalovirus (MCMV) expressing green fluorescent protein (GFP), we tracked viral infection after systemic administration via intraperitoneal injection and showed that specific cells are infected within the first hours. We provide evidence that MCMV traffics as free virus from the peritoneal cavity into the mediastinal lymphatics, providing access to the bloodstream. We demonstrate that MCMV productively infected CD169(+) subcapsular sinus macrophages in the mediastinal lymph nodes, ER-TR7(+) CD29(+) reticular fibroblasts in the spleen and hepatocytes. Infection in the spleen followed a distinctive pattern, beginning in the marginal zone at 6 h and spreading into the red pulp by 17 h. By 48 h after infection, there was widespread infection in the spleen and liver with degeneration of infected cells. In addition, infected dendritic cells appeared in the white pulp of the spleen at 48 h post-infection. On the other hand, cowpox virus showed a different pattern of infectivity in the spleen and liver. Thus, early MCMV infection produces a distinct pattern of infection of selective cells.
Figures
Figure 1
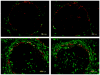
Time course of MCMV infection in the spleen. C57BL/6 mice were infected with 105 pfu MCMV-GFP i.p. and sacrificed at the indicated time points. GFP+ infected cells (green) in the spleen were found in the marginal zone (delineated by MADCAM [red]) by 6h after infection, with increased infection of cells in the red pulp as the infection time was lengthened.
Figure 2
I.p. and i.v. injection of MCMV-GFP produced similar splenic infection patterns. GFP+ infected cells (green) were localized to the MZ (delineated by MADCAM [red]) at 8h p.i. and had spread into the red pulp at 48h p.i. Infected cells had similar morphologies regardless of i.p. vs i.v. infection.
Figure 3
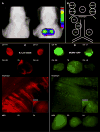
Lymphatic distribution of inert 0.3 μm beads and MCMV-GFP from the peritoneal cavity were similar. (A) Representative digital x-ray images overlaid with NIR fluorescence intensities from a living mouse 1 minute (left) and 6h (right) after i.p. injection of 0.3 μm NIR fluorescent beads are shown. NIR fluorescence was detected from the mediastinum beginning 2h after injection. (B) Schematic diagram of LNs that were sampled for both bead and MCMV-GFP experiments. (C) Removal of LNs demonstrated that beads were contained within mediastinal LNs, particularly the top CM and TB LNs. Other LNs appeared relatively dark due to proportionately smaller number of beads. The diaphragm also contained many beads, in contrast to the control diaphragm which did not. (D) Mice injected with MCMV-GFP i.p. had a distribution of GFP+ MCMV-infected cells similar to the distribution of 0.3 μm beads. GFP+ cells were noted as bright dots in the mediastinal LNs and diaphragm, whereas only background autofluorescence was seen in the abdominal LNs and control diaphragm. LNs and diaphragm shown here were taken from mice sacrificed 24h p.i. CM = cranial mediastinal LN, RT = right top, RB = right bottom, LT = left top, LB = left bottom; TB = tracheobronchial LN; MES = mesenteric LN; PD = pancreaticoduodenal LN; PA = para-aortic LN
Figure 4
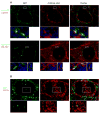
Small beads and MCMV-GFP infected cells preferentially accumulated in the subcapsular sinus of LNs. (A) Sections of mediastinal LNs show accumulation of both 0.3 μm and 1 μm beads in the periphery of the LN. A representative section is shown with 0.3 μm beads. (B) MCMV-GFP infected cells (green) just under the capsule of the LN at 8h p.i. ER-TR7 (red) was used to delineate the capsule. (C) GFP+ infected cells (green) appeared to co-localize with CD169+ (red) macrophages which lie on or just below the floor of the subcapsular sinus. The same image is viewed in green (left), red (middle), and overlay (right) panels.
Figure 5
MCMV-GFP infected reticular fibroblasts at 8h and DCs at 48h in the spleen. (A) GFP+ cells were in close proximity to CD169+ cells, but did not co-localize. Instead, GFP+ cells co-localized with ER-TR7, a marker for reticular fibroblasts. A higher magnification with DAPI nuclear counterstain shows the co-localization more clearly both in the nucleus (rectangle) and cytoplasmic extensions (asterisk). (B) At 48h p.i., infected cells in the white pulp of the spleen followed a distribution similar to that of CD11c+ DCs, and were CD11c+.
Figure 6
Changes in splenic populations were seen at 48h p.i. but not at 8h p.i. At 48h, there was disorganization of T and B cell areas, an influx of CD11b+ macrophages in the red pulp, and loss of NK cells. In addition, ER-TR7 was no longer expressed on many GFP+ cells. In contrast, these populations still appeared histologically normal at 8h.
Figure 7
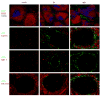
MCMV infected stromal cells in the spleen. Infected cells in the spleen co-localized with ER-TR7 and CD29 at both 8 and 17h p.i. In addition, a few cells appeared to be MADCAM+.
Figure 8
CPXV produced a different pattern of infection in the spleen as compared to MCMV infection. C57BL/6 mice were infected with 5.5×106 pfu CPXV-GFP and sacrificed at 8h p.i. GFP+ cells were found in the MZ and white pulp of the spleen, with few in the red pulp. Unlike MCMV-infected cells, CPXV-infected cells were inside of ER-TR7 staining and there was co-localization with both MARCO and CD169, suggesting that macrophages were infected in the spleen.
Similar articles
- Lymph Node Macrophages Restrict Murine Cytomegalovirus Dissemination.
Farrell HE, Davis-Poynter N, Bruce K, Lawler C, Dolken L, Mach M, Stevenson PG. Farrell HE, et al. J Virol. 2015 Jul;89(14):7147-58. doi: 10.1128/JVI.00480-15. Epub 2015 Apr 29. J Virol. 2015. PMID: 25926638 Free PMC article. - Murine Cytomegalovirus MCK-2 Facilitates In Vivo Infection Transfer from Dendritic Cells to Salivary Gland Acinar Cells.
Ma J, Bruce K, Stevenson PG, Farrell HE. Ma J, et al. J Virol. 2021 Aug 10;95(17):e0069321. doi: 10.1128/JVI.00693-21. Epub 2021 Aug 10. J Virol. 2021. PMID: 34132572 Free PMC article. - Interplay of autophagy and apoptosis during murine cytomegalovirus infection of RPE cells.
Mo J, Zhang M, Marshall B, Smith S, Covar J, Atherton S. Mo J, et al. Mol Vis. 2014 Aug 14;20:1161-73. eCollection 2014. Mol Vis. 2014. PMID: 25324684 Free PMC article. - Developmental disorders of the mouse brain induced by murine cytomegalovirus: animal models for congenital cytomegalovirus infection.
Tsutsui Y. Tsutsui Y. Pathol Int. 1995 Feb;45(2):91-102. doi: 10.1111/j.1440-1827.1995.tb03428.x. Pathol Int. 1995. PMID: 7742931 Review. - Conditional gene expression systems to study herpesvirus biology in vivo.
Sacher T, Jordan S, Mohr CA, Vidy A, Weyn AM, Ruszics Z, Koszinowski UH. Sacher T, et al. Med Microbiol Immunol. 2008 Jun;197(2):269-76. doi: 10.1007/s00430-008-0086-1. Epub 2008 Mar 7. Med Microbiol Immunol. 2008. PMID: 18324415 Review.
Cited by
- Type I Interferons Direct Gammaherpesvirus Host Colonization.
Tan CS, Lawler C, May JS, Belz GT, Stevenson PG. Tan CS, et al. PLoS Pathog. 2016 May 25;12(5):e1005654. doi: 10.1371/journal.ppat.1005654. eCollection 2016 May. PLoS Pathog. 2016. PMID: 27223694 Free PMC article. - Immune surveillance of cytomegalovirus in tissues.
Mihalić A, Železnjak J, Lisnić B, Jonjić S, Juranić Lisnić V, Brizić I. Mihalić A, et al. Cell Mol Immunol. 2024 Sep;21(9):959-981. doi: 10.1038/s41423-024-01186-2. Epub 2024 Aug 12. Cell Mol Immunol. 2024. PMID: 39134803 Free PMC article. Review. - B cell follicles and antigen encounters of the third kind.
Cyster JG. Cyster JG. Nat Immunol. 2010 Nov;11(11):989-96. doi: 10.1038/ni.1946. Epub 2010 Oct 19. Nat Immunol. 2010. PMID: 20959804 Review. - A Review of Murine Cytomegalovirus as a Model for Human Cytomegalovirus Disease-Do Mice Lie?
Fisher MA, Lloyd ML. Fisher MA, et al. Int J Mol Sci. 2020 Dec 28;22(1):214. doi: 10.3390/ijms22010214. Int J Mol Sci. 2020. PMID: 33379272 Free PMC article. Review. - Subcapsular sinus macrophage fragmentation and CD169+ bleb acquisition by closely associated IL-17-committed innate-like lymphocytes.
Gray EE, Friend S, Suzuki K, Phan TG, Cyster JG. Gray EE, et al. PLoS One. 2012;7(6):e38258. doi: 10.1371/journal.pone.0038258. Epub 2012 Jun 1. PLoS One. 2012. PMID: 22675532 Free PMC article.
References
- Andoniou CE, van Dommelen SLH, Voigt V, Andrews DM, Brizard G, Asselin-Paturel C, Delale T, Stacey KJ, Trinchieri G, Degli-Esposti MA. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat Immunol. 2005;6:1011–1019. - PubMed
- Andrews DM, Andoniou CE, Granucci F, Ricciardi-Castagnoli P, Degli-Esposti MA. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat Immunol. 2001;2:1077–1084. - PubMed
- Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. - PubMed
- Backer MV, Levashova Z, Patel V, Jehning BT, Claffey K, Blankenberg FG, Backer JM. Molecular imaging of VEGF receptors in angiogenic vasculature with single-chain VEGF-based probes. Nat Med. 2007;13:504–509. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI057160/AI/NIAID NIH HHS/United States
- AI51345/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- U54 AI057160/AI/NIAID NIH HHS/United States
- R01 AI051345/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources