Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2 - PubMed (original) (raw)
Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2
Susan M Huang et al. J Neurosci. 2008.
Erratum in
- J Neurosci. 2009 Jan 21;29(3):29
Abstract
The ability to sense changes in the environment is essential for survival because it permits responses such as withdrawal from noxious stimuli and regulation of body temperature. Keratinocytes, which occupy much of the skin epidermis, are situated at the interface between the external environment and the body's internal milieu, and have long been appreciated for their barrier function against external insults. The recent discovery of temperature-sensitive transient receptor potential vanilloid (TRPV) ion channels in keratinocytes has raised the possibility that these cells also actively participate in acute temperature and pain sensation. To address this notion, we generated and characterized transgenic mice that overexpress TRPV3 in epidermal keratinocytes under the control of the keratin 14 promoter. Compared with wild-type controls, keratinocytes overexpressing TRPV3 exhibited larger currents as well as augmented prostaglandin E(2) (PGE(2)) release in response to two TRPV3 agonists, 2-aminoethoxydiphenyl borate (2APB) and heat. Thermal selection behavior and heat-evoked withdrawal behavior of naive mice overexpressing TRPV3 were not consistently altered. Upon selective pharmacological inhibition of TRPV1 with JNJ-17203212 [corrected], however, the keratinocyte-specific TRPV3 transgenic mice showed increased escape responses to noxious heat relative to their wild-type littermates. Coadministration of the cyclooxygenase inhibitor, ibuprofen, with the TRPV1 antagonist decreased inflammatory thermal hyperalgesia in transgenic but not wild-type animals. Our results reveal a previously undescribed mechanism for keratinocyte participation in thermal pain transduction through keratinocyte TRPV3 ion channels and the intercellular messenger PGE(2).
Figures
Figure 1.
Generation of keratinocyte-specific TRPV3-overexpressing transgenic mice. A, Keratin 14 promoter driven TRPV3 transgenic constructs. B, Southern blots of _Eco_RV-digested genomic DNA, using the probe indicated in panel A, confirming genomic incorporation of the transgene in 3 lines of transgenic mice (HA-TRPV3 A, HA-TRPV3 B, TRPV3-YFP). T, Transgenic band; E, endogenous band. C–E, Immunoblot comparison of TRPV3 expression in the back skin of adult wild-type mice versus mice from HA-TRPV3 and TRPV3-YFP lines. Blots were probed with mouse monoclonal anti-TRPV3 (C), rat monoclonal anti-HA (D), and rabbit anti-GFP (E). Lane at far left in C shows lysate from HEK293 cells transiently transfected with recombinant TRPV3, whose position is denoted by arrowhead. F, Immunoblots probed with rabbit anti-TRPV3 and rabbit anti-HA antibodies, showing increased transgenic protein expression in cultured HA-TRPV3 B keratinocytes. G, Immunoblots showing increased transgenic protein expression in the skin but not in trigeminal ganglia of TRPV3-YFP mice using rabbit anti-TRPV3 and rabbit anti-GFP antibodies.
Figure 2.
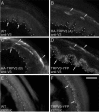
Immunofluorescent localization of TRPV3 in skin of transgenic mice. A–D, Hindpaw glabrous skin of wild-type (A), HA-TRPV3 A (B), HA-TRPV3 B (C), or TRPV3-YFP (D) mice, respectively, probed with anti-TRPV3. Increased TRPV3 staining was observed in the skin epidermis of transgenic mice. E, F, Intrinsic YFP fluorescence in wild-type (E) and TRPV3-YFP transgenic (F) skin. Dashed line denotes dermal-epidermal junction. Arrows mark basal epidermal staining, arrowheads mark suprabasal epidermal staining. Scale bar, 50 μm.
Figure 3.
Whole-cell patch-clamp electrophysiology of primary keratinocytes from TRPV3 transgenic mice. A, Representative current responses of wild-type (top, left) and TRPV3-YFP transgenic (top, right) keratinocytes to stimulation by repeated 42°C heat pulses (bottom) recorded during voltage ramps. Upward current traces were measured at +80 mV and downward traces at −80 mV. B, Representative current traces evoked by 2APB (100 μ
m
) in wild-type (top) and TRPV3-YFP transgenic (bottom) cells. A heat stimulus (41°C) was applied in the middle of the response to 2APB, as indicated by the horizontal bars. C, Quantification of sensitizing current responses to three consecutive heat stimuli (41–43°C) among wild-type and transgenic keratinocytes. Four bars are shown for a given stimulus. The first pair of bars show the mean ± SEM of responses recorded at +80 mV and the right pair show responses at −80 mV. Open bars represents the wild-type response and the filled bars represent the response of the indicated transgenic line. n = 3–21 cells per bar. D, Quantification of current responses evoked by 2APB (100 μ
m
), or the simultaneous application of 2APB plus heat among wild-type (open bars) and transgenic (filled bars) keratinocytes, measured at +80 mV (upward bars) or −80 mV (downward bars). n = 4–21 cells per bar. Progressive increase in response sensitivity to TRPV3 agonists was observed in the order of wild-type < HA-TRPV3 A < HA-TRPV3 B < TRPV3-YFP.
Figure 4.
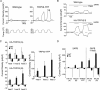
Measurement of PGE2 release from primary keratinocytes. Supernatants from cultured keratinocytes in buffer were collected during baseline and stimulus periods (30 min each), and processed and analyzed for PGE2 using LC-MS/MS. A, TRPV3-YFP transgenic keratinocytes show increased PGE2 release compared with wild-type keratinocytes in response to the TRPV3 agonist 2APB (50–100 μ
m
), but comparable responses to A23187 (1 μ
m
). Inset, Example chromatograms showing detection of PGE2 from buffer under basal and stimulated conditions using LC-MS/MS. B, Exposure to heat (by immersion in a water bath) increased PGE2 release in TRPV3-YFP transgenic keratinocytes more than in wild type. C, Supra-additive increase in stimulated PGE2 release in HA-TRPV3 line B compared with wild-type keratinocytes in response to coapplication of 2APB (100 μ
m
) and 36°C. D, Cumulative stimulated PGE2 release over time (buffer collected after 1, 5, 10 or 30 min incubation) in wild-type and HA-TRPV3 line B keratinocytes. E, 2APB (100 μ
m
) plus 36°C-stimulated PGE2 release in HA-TRPV3 line B keratinocytes is diminished in calcium-free buffer and in the presence of COX antagonists. Data are expressed as mean ± SEM, n = 3 per group. A–D were analyzed using ANOVA with Bonferroni post hoc comparisons and E was analyzed using ANOVA with Dunnett's test. **p < 0.001, *p < 0.01, difference between wild type and transgenic (A–D) or between pharmacological treatment and vehicle (DMSO) control in cells treated with 2APB plus heat (E).
Figure 5.
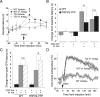
Temperature preference and pain behavior of wild-type and keratinocyte TRPV3 overexpressing mice. A, Temperature preference behavior of HA-TRPV3 line B mice (top) and TRPV3-YFP mice (bottom) over 60–120 min after placement in a temperature gradient ranging from 0 to 49°C. Mean presence time at each temperature is illustrated. B, Tail withdrawal behavior of HA-TRPV3 line B mice (top) and TRPV3-YFP mice (bottom) exposed to a heated water bath. C, Latency of paw withdrawal from radiant heat in wild-type and TRPV3-YFP mice before and 24 h after injection of CFA (15 μl, intraplantar) into the ipsilateral hindpaw. D, Latency of HA-TRPV3 line B (top), HA-TRPV3 line A (middle) and TRPV3-YFP (bottom) mice to paw licking, biting, or shaking behavior upon placement on a hot plate at the indicated temperatures. In each graph, data shown are from a single cohort of 9–13 mice per genotype. Two of three cohorts of HA-TRPV3 B mice, one of two cohorts of HA-TRPV3 A mice and two of two cohorts of TRPV3-YFP mice tested showed the presented response (see also supplemental Fig. S4_C_, available at
as supplemental material). Hot plate data were analyzed using Mann–Whitney U test. **p < 0.0001, *p < 0.05. Data are represented as mean ± SEM. Numbers of mice (n) are indicated in each panel.
Figure 6.
Changes in thermal pain behavior of TRPV3-YFP transgenic mice unmasked by pharmacological antagonism of TRPV1. A, Hot plate (52°C) latencies of wild-type and TRPV3-YFP transgenic mice before and after administration of the TRPV1 antagonist JNJ-7203212 (40 mg/kg, injected i.p. at arrow). Data were analyzed by ANOVA with Bonferroni post hoc comparisons; *p < 0.05 antagonist versus vehicle; #p < 0.05 wild type versus transgenic. B, Effects of cyclooxygenase antagonism. Wild-type and TRPV3-YFP transgenic mice were pretreated with the COX antagonist ibuprofen (40 mg/kg, i.p.) or vehicle 15 min before injection of JNJ-7203212 or vehicle. Beginning 30 min after the final injection, hot plate latency was measured at 30 min intervals and 6 consecutive values averaged to calculate difference from the baseline (measured during the 90 min before ibuprofen injection). C, Effects of TRPV1 and COX antagonists on CFA-induced thermal hyperalgesia in wild-type and TRPV3-YFP transgenic mice. Immediately following the measurement of post-CFA paw withdrawal latency (Fig. 5_C_), TRPV3-YFP transgenic and wild-type mice were injected with ibuprofen (i.p., 40 mg/kg) or vehicle, and 15 min later injected with JNJ-7203212 (40 mg/kg, i.p.) or vehicle. After an additional 30 min, latency to escape from radiant paw heating was measured at 20 min intervals in the ipsilateral paw. The three latencies obtained between 50 and 90 min postinjection were averaged to calculate the change from the post-CFA baseline measured before ibuprofen injection. Note that ibuprofen increases withdrawal latency in TRPV3-YFP, but not wild-type mice treated with the TRPV1 antagonist. Planned comparisons of data in B and C were analyzed by Student's t test. *p < 0.05; n.s., no significant difference (n = 6–12 per group). D, Change in core body temperature in response to injection of JNJ-7203212 (40 mg/kg, i.p.) in wild-type (vehicle n = 4, JNJ-7203212 n = 5) versus TRPV3-YFP (JNJ-7203212; n = 8) mice.
Similar articles
- 2-aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3.
Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. Chung MK, et al. J Neurosci. 2004 Jun 2;24(22):5177-82. doi: 10.1523/JNEUROSCI.0934-04.2004. J Neurosci. 2004. PMID: 15175387 Free PMC article. - TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation.
Huang SM, Li X, Yu Y, Wang J, Caterina MJ. Huang SM, et al. Mol Pain. 2011 May 17;7:37. doi: 10.1186/1744-8069-7-37. Mol Pain. 2011. PMID: 21586160 Free PMC article. - Sorting nexin 11 knockout mice exhibit enhanced thermosensing behaviour.
Huang HL, Li C, Ma W, Yin S, Zhao H, Deng S, Shu X, Wu D, Li J, Huang R, Cheng N, Huang J, Li Z. Huang HL, et al. Genes Brain Behav. 2020 Jul;19(6):e12625. doi: 10.1111/gbb.12625. Epub 2019 Dec 15. Genes Brain Behav. 2020. PMID: 31730264 Review. - A TR(I)P to pruritus research: role of TRPV3 in inflammation and itch.
Steinhoff M, Bíró T. Steinhoff M, et al. J Invest Dermatol. 2009 Mar;129(3):531-5. doi: 10.1038/jid.2008.440. J Invest Dermatol. 2009. PMID: 19209153 Review.
Cited by
- The thermo-TRP ion channel family: properties and therapeutic implications.
Vay L, Gu C, McNaughton PA. Vay L, et al. Br J Pharmacol. 2012 Feb;165(4):787-801. doi: 10.1111/j.1476-5381.2011.01601.x. Br J Pharmacol. 2012. PMID: 21797839 Free PMC article. Review. - Acute heat-evoked temperature sensation is impaired but not abolished in mice lacking TRPV1 and TRPV3 channels.
Marics I, Malapert P, Reynders A, Gaillard S, Moqrich A. Marics I, et al. PLoS One. 2014 Jun 12;9(6):e99828. doi: 10.1371/journal.pone.0099828. eCollection 2014. PLoS One. 2014. PMID: 24925072 Free PMC article. - Resolvin D1 attenuates activation of sensory transient receptor potential channels leading to multiple anti-nociception.
Bang S, Yoo S, Yang TJ, Cho H, Kim YG, Hwang SW. Bang S, et al. Br J Pharmacol. 2010 Oct;161(3):707-20. doi: 10.1111/j.1476-5381.2010.00909.x. Br J Pharmacol. 2010. PMID: 20880407 Free PMC article. - Nociceptors: the sensors of the pain pathway.
Dubin AE, Patapoutian A. Dubin AE, et al. J Clin Invest. 2010 Nov;120(11):3760-72. doi: 10.1172/JCI42843. Epub 2010 Nov 1. J Clin Invest. 2010. PMID: 21041958 Free PMC article. Review. - TRPV3 modulates nociceptive signaling through peripheral and supraspinal sites in rats.
McGaraughty S, Chu KL, Xu J, Leys L, Radek RJ, Dart MJ, Gomtsyan A, Schmidt RG, Kym PR, Brederson JD. McGaraughty S, et al. J Neurophysiol. 2017 Aug 1;118(2):904-916. doi: 10.1152/jn.00104.2017. Epub 2017 May 3. J Neurophysiol. 2017. PMID: 28468993 Free PMC article.
References
- Albers KM, Davis BM. The skin as a neurotrophic organ. Neuroscientist. 2007;13:371–382. - PubMed
- Asakawa M, Yoshioka T, Matsutani T, Hikita I, Suzuki M, Oshima I, Tsukahara K, Arimura A, Horikawa T, Hirasawa T, Sakata T. Association of a mutation in TRPV3 with defective hair growth in rodents. J Invest Dermatol. 2006;126:2664–2672. - PubMed
- Birder L, Kullmann FA, Lee H, Barrick S, de Groat W, Kanai A, Caterina M. Activation of urothelial transient receptor potential vanilloid 4 by 4alpha-phorbol 12,13-didecanoate contributes to altered bladder reflexes in the rat. J Pharmacol Exp Ther. 2007;323:227–235. - PubMed
- Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856–860. - PubMed
- Bley KR, Hunter JC, Eglen RM, Smith JA. The role of IP prostanoid receptors in inflammatory pain. Trends Pharmacol Sci. 1998;19:141–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS051551-03/NS/NINDS NIH HHS/United States
- R01 NS051551/NS/NINDS NIH HHS/United States
- R01 NS051551-01/NS/NINDS NIH HHS/United States
- NS051551/NS/NINDS NIH HHS/United States
- R01 NS051551-04/NS/NINDS NIH HHS/United States
- AR44232/AR/NIAMS NIH HHS/United States
- R01 AR044232/AR/NIAMS NIH HHS/United States
- DA-018244/DA/NIDA NIH HHS/United States
- R01 NS051551-02/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases