Dendrites of mammalian neurons contain specialized P-body-like structures that respond to neuronal activation - PubMed (original) (raw)
Dendrites of mammalian neurons contain specialized P-body-like structures that respond to neuronal activation
Nicolas Cougot et al. J Neurosci. 2008.
Abstract
Intracellular mRNA transport and local translation play a key role in neuronal physiology. Translationally repressed mRNAs are transported as a part of ribonucleoprotein (RNP) particles to distant dendritic sites, but the properties of different RNP particles and mechanisms of their repression and transport remain largely unknown. Here, we describe a new class of RNP-particles, the dendritic P-body-like structures (dlPbodies), which are present in the soma and dendrites of mammalian neurons and have both similarities and differences to P-bodies of non-neuronal cells. These structures stain positively for a number of P-body and microRNP components, a microRNA-repressed mRNA and some translational repressors. They appear more heterogeneous than P-bodies of HeLa cells, and they rarely contain the exonuclease Xrn1 but are positive for rRNA. These particles show motorized movements along dendrites and relocalize to distant sites in response to synaptic activation. Furthermore, Dcp1a is stably associated with dlP-bodies in unstimulated cells, but exchanges rapidly on neuronal activation, concomitantly with the loss of Ago2 from dlP-bodies. Thus, dlP-bodies may regulate local translation by storing repressed mRNPs in unstimulated cells, and releasing them on synaptic activation.
Figures
Figure 1.
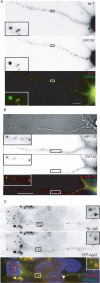
A–E, Dendritic localization of P-body-like structures in rat hippocampal and hypothalamic neurons. In vitro cultured rat hypothalamic (B–D) or hippocampal (A) neurons were used for immunofluorescence analysis to study the distribution of several P-body markers. In A–D, scale bars represent 10 μm. The insets show enlargements of indicated regions (4×). A, Costaining of hippocampal neurons with anti-GW182 (bottom) and anti-PSD95 (middle; a marker for synapses) antibodies. GW182-positive granule present close to PSD95 positive synaptic site is marked by an arrow. Overlay (colored panel): GW182 staining is in green and PSD95 in red. DIC image of the same dendrite is shown in the top. B, Costaining of hypothalamic neurons with anti-Dcp1a (top) and anti-Ago2 (middle). Overlay (bottom): Dcp1a staining in green, Ago2 in red. C, Foci that are stained with anti-Dcp1a serum (left) were also labeled with anti-RCK/p54 antibodies (middle) in processes of cultured hypothalamic neurons. Overlay (right): Dcp1a is in green and rck/p54 is in red. D, Anti-Dcp1a antibody (left) labels foci in dendrites of rat hypothalamic neurons. Neurons were costained for neuronal dendritic marker MAP2 (middle). On the overlay (right) panel, Dcp1a is in green and the MAP2 staining is in red. Nuclei are stained with DAPI (in blue). E, Western blot reveal the presence of Dcp1a in crude and purified synaptosomal fractions prepared from the mouse brain extract.
Figure 2.
Localization of miRNAs and their mRNA targets in dlP-bodies. A, B, Colocalization of miRNAs and GW182 in dlP-bodies of hypothalamic (A) and hippocampal (B) neurons. miRNAs let-7 (A) or miR-128 (B) were detected by in situ hybridization (top). Staining with anti-GW182 antibody is shown in middle panels. Overlay pictures are shown in bottom, with staining for miRNA in red and for GW182 in green. C, Hypothalamic neurons were cotransfected with plasmids expressing CFP-Ago2 (middle) and a Renilla luciferase (RL) mRNA reporter with three let-7 binding sites in its 3′ UTR (RL-3xB; top). In situ hybridization, using probes complementary to RL mRNA, detected the mRNA (red) in foci that are also positive for CFP-Ago2 (green; bottom). Scale bar, 10 μm. The insets show 4× enlargements of indicated areas. DAPI was used for staining the nuclei.
Figure 3.
dlP-bodies contain translational repressor proteins. A, Anti-ZBP1 (top) and anti-GW182 (middle) antibody were used for detection of endogenous proteins in hippocampal neurons. On the overlay (bottom) panel, ZBP1 is in red, and GW182 in green. Colocalizations in panel (A–D) are marked by arrowheads. Scale bars, 10 μm. The insets show 4× enlargements of indicated regions. DAPI was used for staining the nuclei. B, Hypothalamic neurons transfected with CFP-Dcp1a (top) and YFP-ZBP1 (middle) show colocalization of CFP and YFP signals in discrete foci. Overlay (lower) panel shows colocalization of CFP-Dcp1 (red) with YFP-ZBP1 (green). C, GFP-tagged FMRP (middle) colocalizes with the endogenous Dcp1a (top) in dlP-bodies of hypothalamic neurons transfected with the GFP-FMRP expression plasmid. Overlay (bottom) panel shows colocalization of Dcp1a (red) with GFP-FMRP (green). D, Localization of SMN and Dcp1a proteins in hypothalamic neurons. Neurons expressing SMN-RFP (middle) were labeled with anti-Dcp1a (top). In overlay panel (bottom) Dcp1a is in green and SMN-RFP in red. E, ZBP1 and RCK/p54 coimmunoprecipitate with each other in protein extracts prepared from purified synaptosomes. Input represents 10% of the total extract used for each immunoprecipitation reaction. Rabbit polyclonal antibody against GRP78 protein was used in control immunoprecipitation.
Figure 4.
dlP-bodies rarely contain the exonuclease Xrn1, but contain rRNA. A, Localization of Xrn1 (middle panel) and Dcp1a (top) in hypothalamic neurons transfected with a GFP-Dcp1a expression plasmid. The overlay (bottom) shows Xrn1 staining in red, GFP-Dcp1a in green and nuclei, stained with DAPI, in blue. A rabbit anti-Xrn1 antibody was used for detection of endogenous Xrn1. Insets (2× enlargements of the selected areas) show localization of both proteins in soma (left insets) and dendrites (right insets). B, Colocalization of rRNA (staining with Y10B mAb in top; red signal in the overlay panel) with anti-GW182 antibodies (middle; green in the overlay) in hippocampal neurons. Insets show 4× enlargements of the selected areas. C, D, Localization of rRNA (C) or Xrn1(D) in HeLa cells (top; red in the overlay panel) transfected with GFP-Dcp1a (middle panels; green in the overlay). Nuclei are stained with DAPI (blue in the overlay). Scale bars: 10 μm (in A, B); 5 μm (in C, D).
Figure 5.
P-body components show directed and motorized movements along dendrites of rat neurons. Hypothalamic neurons transfected with GFP-Ago2 (A), GFP-Dcp1a (B) or GFP-SMN (C) were analyzed by time-lapse microscopy. Insets show enlargements (5× for GFPAgo2, 60× for GFP-Dcp1a, and 11× for GFP-SMN) of pictures taken at 250 ms intervals. Asterisks indicate initial position of foci, and arrows indicate their position at the indicated time.
Figure 6.
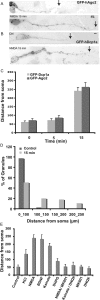
A, B, Treatment with NMDA and other synaptic activators relocates dlP-bodies to distant parts of dendrites of rat neuron. Localization of GFP-Ago2 (A) and GFP-Dcp1a (B) positive foci in hypothalamic neurons either untreated (Ctrl) or after 15 min treatment with 30 μ
m
NMDA. C, Quantification of the distance between dlP-bodies and the cell body. GFP-Ago2 (dark gray) and GFP-Dcp1a (light gray) in untreated neurons and in neurons treated with NMDA for 5 or 15 min. The numbers represent the maximum distance from cell soma in which GFP-Ago2 or GFP-Dcp1a foci were detectable. D, Distribution of GFP-Ago2 positive foci along dendrites before and after 15 min NMDA treatment (n = 11). E, Quantification of dendritic distribution of GFP-Dcp1a after 15 min treatment with different glutamatergic receptor agonists: NMDA (30 μ
m
), kainate (50 μ
m
) or DHPG (50 μ
m
), or BDNF (50 mg/ml) and KCl (50 m
m
). When indicated, specific antagonists of NMDA and kainate, MK-801 (10 μ
m
) and DNQX (50 μ
m
), respectively, were added to the culture medium. The numbers the maximum distance from cell soma in which GFP-Ago2 or GFP-Dcp1a foci were detectable after different treatments (n = 14).
Figure 7.
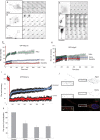
Synaptic stimulation modifies the exchange rates of of dlP-bodies components and modifies their composition. Hypothalamic neurons and HeLa cells expressing GFP-hDcp1a (A–C) or GFP-Ago2 (E–G) were analyzed by FRAP. Specific foci were bleached after 30 s recording, and fluorescent signals were recorded over time. Images of individual cells and enlargement of the bleached foci is shown. The graphs show recovery curves against time for GFPhDcp1a foci (D) or Ago2 foci (H). I, FRAP analysis of GFP-hDcp1 in dendrites before (blue) or after stimulation with 30 μ
m
NMDA (red). J, Hypothalamic neurons were stimulated (30 μ
m
NMDA for 15 min), and analyzed by immunofluorescence with antibodies against Ago2 and hDcp1a. K, Quantification of the fraction of Dcp1a foci that contained Ago2.
Similar articles
- Dynamic interaction between P-bodies and transport ribonucleoprotein particles in dendrites of mature hippocampal neurons.
Zeitelhofer M, Karra D, Macchi P, Tolino M, Thomas S, Schwarz M, Kiebler M, Dahm R. Zeitelhofer M, et al. J Neurosci. 2008 Jul 23;28(30):7555-62. doi: 10.1523/JNEUROSCI.0104-08.2008. J Neurosci. 2008. PMID: 18650333 Free PMC article. - Staufen- and FMRP-containing neuronal RNPs are structurally and functionally related to somatic P bodies.
Barbee SA, Estes PS, Cziko AM, Hillebrand J, Luedeman RA, Coller JM, Johnson N, Howlett IC, Geng C, Ueda R, Brand AH, Newbury SF, Wilhelm JE, Levine RB, Nakamura A, Parker R, Ramaswami M. Barbee SA, et al. Neuron. 2006 Dec 21;52(6):997-1009. doi: 10.1016/j.neuron.2006.10.028. Neuron. 2006. PMID: 17178403 Free PMC article. - Dendritic LSm1/CBP80-mRNPs mark the early steps of transport commitment and translational control.
di Penta A, Mercaldo V, Florenzano F, Munck S, Ciotti MT, Zalfa F, Mercanti D, Molinari M, Bagni C, Achsel T. di Penta A, et al. J Cell Biol. 2009 Feb 9;184(3):423-35. doi: 10.1083/jcb.200807033. Epub 2009 Feb 2. J Cell Biol. 2009. PMID: 19188494 Free PMC article. - How voltage-gated ion channels alter the functional properties of ganglion and amacrine cell dendrites.
Miller RF, Stenback K, Henderson D, Sikora M. Miller RF, et al. Arch Ital Biol. 2002 Oct;140(4):347-59. Arch Ital Biol. 2002. PMID: 12228988 Review. - Dendritic attenuation of synaptic potentials and currents: the role of passive membrane properties.
Spruston N, Jaffe DB, Johnston D. Spruston N, et al. Trends Neurosci. 1994 Apr;17(4):161-6. doi: 10.1016/0166-2236(94)90094-9. Trends Neurosci. 1994. PMID: 7517596 Review.
Cited by
- Rotavirus Induces Formation of Remodeled Stress Granules and P Bodies and Their Sequestration in Viroplasms To Promote Progeny Virus Production.
Dhillon P, Rao CD. Dhillon P, et al. J Virol. 2018 Nov 27;92(24):e01363-18. doi: 10.1128/JVI.01363-18. Print 2018 Dec 15. J Virol. 2018. PMID: 30258011 Free PMC article. - Conformational dynamics in the disordered region of human CPEB3 linked to memory consolidation.
Ramírez de Mingo D, Pantoja-Uceda D, Hervás R, Carrión-Vázquez M, Laurents DV. Ramírez de Mingo D, et al. BMC Biol. 2022 Jun 3;20(1):129. doi: 10.1186/s12915-022-01310-6. BMC Biol. 2022. PMID: 35658951 Free PMC article. - RNA granules: the good, the bad and the ugly.
Thomas MG, Loschi M, Desbats MA, Boccaccio GL. Thomas MG, et al. Cell Signal. 2011 Feb;23(2):324-34. doi: 10.1016/j.cellsig.2010.08.011. Epub 2010 Sep 8. Cell Signal. 2011. PMID: 20813183 Free PMC article. Review. - Quantifying Argonaute proteins in and out of GW/P-bodies: implications in microRNA activities.
Leung AK, Sharp PA. Leung AK, et al. Adv Exp Med Biol. 2013;768:165-82. doi: 10.1007/978-1-4614-5107-5_10. Adv Exp Med Biol. 2013. PMID: 23224970 Free PMC article. Review. - Role of a VGF/BDNF/TrkB Autoregulatory Feedback Loop in Rapid-Acting Antidepressant Efficacy.
Jiang C, Lin WJ, Salton SR. Jiang C, et al. J Mol Neurosci. 2019 Jul;68(3):504-509. doi: 10.1007/s12031-018-1124-0. Epub 2018 Jul 18. J Mol Neurosci. 2019. PMID: 30022437 Free PMC article. Review.
References
- Antar LN, Dictenberg JB, Plociniak M, Afroz R, Bassell GJ. Localization of FMRP-associated mRNA granules and requirement of microtubules foractivity-dependent trafficking in hippocampal neurons. Genes Brain Behav. 2005;4:350–359. - PubMed
- Ashraf SI, Kunes S. A trace of silence: memory and microRNA at the synapse. Curr Opin Neurobiol. 2006;16:535–539. - PubMed
- Barbee SA, Estes PS, Cziko AM, Hillebrand J, Luedeman RA, Coller JM, Johnson N, Howlett IC, Geng C, Ueda R, Brand AH, Newbury SF, Wilhelm JE, Levine RB, Nakamura A, Parker R, Ramaswami M. Staufen- and FMRPcontaining neuronal RNPs are structurally and functionally related to somatic P bodies. Neuron. 2006;52:997–1009. - PMC - PubMed
- Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125:1111–1124. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases