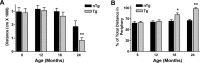Amyloid pathology is associated with progressive monoaminergic neurodegeneration in a transgenic mouse model of Alzheimer's disease - PubMed (original) (raw)
Amyloid pathology is associated with progressive monoaminergic neurodegeneration in a transgenic mouse model of Alzheimer's disease
Ying Liu et al. J Neurosci. 2008.
Abstract
beta-Amyloid (Abeta) pathology is an essential pathogenic component in Alzheimer's disease (AD). However, the significance of Abeta pathology, including Abeta deposits/oligomers and glial reactions, to neurodegeneration is unclear. In particular, despite the Abeta neurotoxicity indicated by in vitro studies, mouse models with significant Abeta deposition lack robust and progressive loss of forebrain neurons. Such results have fueled the view that Abeta pathology is insufficient for neurodegeneration in vivo. In this study, because monoaminergic (MAergic) neurons show degenerative changes at early stages of AD, we examined whether the APPswe/PS1DeltaE9 mouse model recapitulates progressive MAergic neurodegeneration occurring in AD cases. We show that the progression forebrain Abeta deposition in the APPswe/PS1DeltaE9 model is associated with progressive losses of the forebrain MAergic afferents. Significantly, axonal degeneration is associated with significant atrophy of cell bodies and eventually leads to robust loss (approximately 50%) of subcortical MAergic neurons. Degeneration of these neurons occurs without obvious local Abeta or tau pathology at the subcortical sites and precedes the onset of anxiety-associated behavior in the mice. Our results show that a transgenic mouse model of Abeta pathology develops progressive MAergic neurodegeneration occurring in AD cases.
Figures
Figure 1.
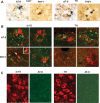
Progressive Aβ deposition in APPswe/PS1_Δ_E9 mice. A–C, Coronal brain sections from 4-, 12-, and 18-month-old APPswe/PS1_Δ_E9 Tg mice immunostained for Aβ (antibody 4G8). An increase in the Aβ deposits with aging is apparent in cortex (Ctx) and hippocampus (CA1; DG, dentate gyrus). Few Aβ deposits (A, arrows) are seen at 4 months of age. mos, Months. D–F, Aβ immunostaining of brain sections from 18-month-old APPswe/PS1_Δ_E9 mice containing ventral midbrain (D; SNr, substantia nigra pars reticulate), dorsal raphe (DR; E), and LC (F). Except for a few Aβ deposits (arrows in D and F), these structures are relatively free of Aβ deposits. Aq, Aquaduct; Cblm, cerebellum; IV, fourth ventricle.
Figure 2.
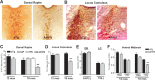
MAergic axonal dystrophy and degeneration in APPswe/PS1_Δ_E9 mice. A, Double labeling of cortex for 5-HT+/TH+ axons (brown) with Aβ deposits (antibody 4G8, blue) in 4- and 12-month-old APPswe/PS1_Δ_E9 mice showing progressive axonal dystrophy with aging. B, C, Dark-field images of cortical 5-HT+ and TH+ axons at 4 months of age (B) and 18 months of age (C). Shown are representative images of non-Tg (nTg), singly transgenic (APP or PS), and APPswe/PS1_Δ_E9 (APP/PS) mice. mos, Months.
Figure 3.
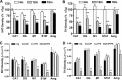
Progressive losses of MAergic axons with aging in the APPswe/PS1_Δ_E9 mice. A, B, The densities of 5-HT+ (A) and TH+ (B) axons in hippocampus (CA1 and dentate gyrus), motor cortex (M1), somatosensory cortex-barrel field (S1bf), and amygdala in 4-month-old (4m), 12-month-old (12m), and 18-month-old (18m) APPswe/PS1_Δ_E9 mice. The axon density was normalized to the mean density of age-matched non-Tg mice (n = 4–8 mice per group; **p < 0.01 vs non-Tg mice). C, D, Quantitative analysis of 5-HT+ (A) and TH+ (B) axon densities in hippocampus (CA1, dentate gyrus), motor cortex (M1), barrel cortex (S1bf), and amygdala of 18-month-old non-Tg, _APPswe_-alone (AP), _PS1_Δ_E9_-alone (PS), and APPswe/PS1_Δ_E9 (AP/PS) mice. Only the AP/PS mice show significantly (**p < 0.01; n = 4–6 mice per group) lower densities of MAergic axons compared with non-Tg mice. Error bars show SEM. DG, Dentate gyrus; Amg, amygdale; nTg, non-Tg.
Figure 4.
Loss of MAergic neurons in APPswe/PS1_Δ_E9 mice. A, B, Representative images of dorsal raphe (A), immunostained for 5-HT+ neurons, and LC (B), immunostained for TH+ neurons, from 18-month-old non-Tg and APPswe/PS1_Δ_E9 (AP/PS) mice. C, D, Stereological estimate of 5-HT+ neuron number in dorsal raphe (C) and TH+ neuron number in LC (D) of 12- and 18-month-old non-Tg and Tg mice. Only the 18-month-old AP/PS mice show significant loss of MAergic neurons (n = 4–8 per group; **p < 0.01 vs non-Tg mice). Analysis of median raphe also showed similar loss of neurons (data not shown). E, Stereological estimates of the number of Nissl-stained 5-HT(−) and TH(−) neurons in dorsal raphe (DR) and LC, respectively, of 18-month-old non-Tg and AP/PS mice. Loss of neuronal phenotype without cell loss would lead to increased number of 5-HT(−) and TH(−) neurons. F, The numbers of TH+ dopaminergic neurons in SNpc and VTA in 12- and 18-month-old non-Tg and AP/PS mice. There is a significant loss of TH+ dopaminergic neurons in VTA of 18-month-old AP/PS mice (n = 4–8 per group; **p < 0.01). Symbol designations are as shown in C. Error bars show SEM. non-Tg, Non-Tg.
Figure 5.
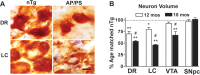
Atrophy of MA neurons precedes cell loss in APPswe/PS1_Δ_E9 mice. A, High-magnification images of 5-HT+ neurons in dorsal raphe and TH+ neurons in LC from 18-month-old non-Tg and APPswe/PS1_Δ_E9 (AP/PS) mice. Scale bar, 20 μm. B, Average volumes of 5-HT (dorsal raphe), NA (LC), and DA (VTA and SNpc) neurons from 12- and 18-month-old APPswe/PS1_Δ_E9 subjects. Shown are percentages of neuronal volumes of age-matched non-Tg mice (n = 4–6 mice per group; **p < 0.01 or *p < 0.05 vs non-Tg mice; #p < 0.05 vs 12-month-old AP/PS mice). Error bars show SEM. Actual neuronal volumes are shown in supplemental table S1. DR, dorsal raphe; nTg, non-Tg; mos, months.
Figure 6.
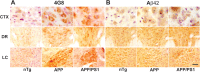
MAergic neurodegeneration occurs without accumulation of intracellular Aβ in MAergic neurons. Brain sections from 8-month-old non-Tg (nTg), APPswe (APP), and APPswe/PS1_Δ_E9 (APP/PS1) mice, containing cortex (CTX; scale bar, 50 μm), dorsal raphe (DR; scale bar, 100 μm), and LC (scale bar, 100 μm), were immunostained using the 4G8 antibody (A) or an anti-Aβ42 antibody (B). Note the increased 4G8 staining in the Tg mice. In contrast, anti-Aβ42 immunoreactivity in Tg mice is not notably different from nTg mice. The 4G8 immunoreactivity is very similar between APP and APP/PS1 mice.
Figure 7.
MAergic neurodegeneration is not associated with accumulation of phosphorylated tau in MAergic neurites or neurons. A, Brain sections double immunostained for phosphorylated tau (AT-8 or PHF1; brown) and MAergic axons (5-HT or TH; blue). Both phosphorylated tau and dystrophic MAergic axons localize around the amyloid deposits. B, Confocal immunofluorescence analysis of sections from APPswe/PS1_Δ_E9 mice (12 months old) reacted for phosphorylated tau (AT-8 or PHF1; green) and MAergic axons (5-HT or TH; red). Stacked images reconstructed from 10–16 optical sections are shown. The images show that very little, if any, overlap of phosphorylated tau with the dystrophic MAergic axons. The inset shows 180° rotation of the area indicated by the arrow. The areas of overlap (yellow) result as artifacts of “transparency” settings for reconstruction and do not represent coincidence within the same optical section(s). C, Although we are able to clearly document phosphorylated tau associated with amyloid deposits (A, B), we could not document neuronal staining. Double-immunofluorescence labeling of phosho-tau (AT8; green) with 5-HT (red) or TH (red) shows lack of significant AT-8 immunoreactivity in MAergic neurons.
Figure 8.
Increased anxiety-related behaviors in aged APPswe/PS1_Δ_E9 mice. A, Reduced novelty-induced exploration in APPswe/PS1_Δ_E9 mice. The motor activity in the open-field test (total distance traveled in centimeters ± SEM) is lower in 24-month-old Tg mice. B, Increased thigmotaxis in APPswe/PS1_Δ_E9 mice. The values are percentages (±SEM) of total distance traveled (A) within the periphery of the activity box. All values are mean ± SEM (*p < 0.05; **p < 0.01; n = 8–12 mice per group). nTg, Non-Tg.
Similar articles
- Passive (amyloid-β) immunotherapy attenuates monoaminergic axonal degeneration in the AβPPswe/PS1dE9 mice.
Liu Y, Lee MK, James MM, Price DL, Borchelt DR, Troncoso JC, Oh ES. Liu Y, et al. J Alzheimers Dis. 2011;23(2):271-9. doi: 10.3233/JAD-2010-101602. J Alzheimers Dis. 2011. PMID: 20966549 Free PMC article. - Cholinergic forebrain degeneration in the APPswe/PS1DeltaE9 transgenic mouse.
Perez SE, Dar S, Ikonomovic MD, DeKosky ST, Mufson EJ. Perez SE, et al. Neurobiol Dis. 2007 Oct;28(1):3-15. doi: 10.1016/j.nbd.2007.06.015. Epub 2007 Jun 27. Neurobiol Dis. 2007. PMID: 17662610 Free PMC article. - The cholinergic system in aging and neuronal degeneration.
Schliebs R, Arendt T. Schliebs R, et al. Behav Brain Res. 2011 Aug 10;221(2):555-63. doi: 10.1016/j.bbr.2010.11.058. Epub 2010 Dec 9. Behav Brain Res. 2011. PMID: 21145918 Review. - Always around, never the same: pathways of amyloid beta induced neurodegeneration throughout the pathogenic cascade of Alzheimer's disease.
Hoozemans JJ, Chafekar SM, Baas F, Eikelenboom P, Scheper W. Hoozemans JJ, et al. Curr Med Chem. 2006;13(22):2599-605. doi: 10.2174/092986706778201585. Curr Med Chem. 2006. PMID: 17017913 Review.
Cited by
- Microglia Express Insulin-Like Growth Factor-1 in the Hippocampus of Aged APPswe/PS1ΔE9 Transgenic Mice.
Myhre CL, Thygesen C, Villadsen B, Vollerup J, Ilkjær L, Krohn KT, Grebing M, Zhao S, Khan AM, Dissing-Olesen L, Jensen MS, Babcock AA, Finsen B. Myhre CL, et al. Front Cell Neurosci. 2019 Jul 30;13:308. doi: 10.3389/fncel.2019.00308. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31417357 Free PMC article. - γ-Glutamyl-Transpeptidase-Resistant Glutathione Analog Attenuates Progression of Alzheimer's Disease-like Pathology and Neurodegeneration in a Mouse Model.
Christopher Kwon YI, Xie W, Zhu H, Xie J, Shinn K, Juckel N, Vince R, More SS, Lee MK. Christopher Kwon YI, et al. Antioxidants (Basel). 2021 Nov 10;10(11):1796. doi: 10.3390/antiox10111796. Antioxidants (Basel). 2021. PMID: 34829667 Free PMC article. - Loss of tau expression attenuates neurodegeneration associated with α-synucleinopathy.
Vermilyea SC, Christensen A, Meints J, Singh B, Martell-Martínez H, Karim MR, Lee MK. Vermilyea SC, et al. Transl Neurodegener. 2022 Jul 1;11(1):34. doi: 10.1186/s40035-022-00309-x. Transl Neurodegener. 2022. PMID: 35773715 Free PMC article. - Intravenous ascorbate improves spatial memory in middle-aged APP/PSEN1 and wild type mice.
Kennard JA, Harrison FE. Kennard JA, et al. Behav Brain Res. 2014 May 1;264:34-42. doi: 10.1016/j.bbr.2014.01.044. Epub 2014 Feb 5. Behav Brain Res. 2014. PMID: 24508240 Free PMC article. - Molecular imaging of neuropsychiatric symptoms in Alzheimer's and Parkinson's disease.
Hirao K, Pontone GM, Smith GS. Hirao K, et al. Neurosci Biobehav Rev. 2015 Feb;49:157-70. doi: 10.1016/j.neubiorev.2014.11.010. Epub 2014 Nov 20. Neurosci Biobehav Rev. 2015. PMID: 25446948 Free PMC article. Review.
References
- Calhoun ME, Wiederhold KH, Abramowski D, Phinney AL, Probst A, Sturchler-Pierrat C, Staufenbiel M, Sommer B, Jucker M. Neuron loss in APP transgenic mice. Nature. 1998;395:755–756. - PubMed
- Coleman PD, Yao PJ. Synaptic slaughter in Alzheimer's disease. Neurobiol Aging. 2003;24:1023–1027. - PubMed
- Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835:18–26. - PubMed
- Fischer LR, Culver DG, Tennant P, Davis AA, Wang MS, Castellano-Sanchez A, Khan J, Polak MA, Glass JD. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous