Regeneration of glomerular podocytes by human renal progenitors - PubMed (original) (raw)
doi: 10.1681/ASN.2008070709. Epub 2008 Dec 17.
Costanza Sagrinati, Maria Lucia Angelotti, Elena Lazzeri, Benedetta Mazzinghi, Lara Ballerini, Eliana Parente, Francesca Becherucci, Mauro Gacci, Marco Carini, Enrico Maggi, Mario Serio, Gabriella Barbara Vannelli, Laura Lasagni, Sergio Romagnani, Paola Romagnani
Affiliations
- PMID: 19092120
- PMCID: PMC2637058
- DOI: 10.1681/ASN.2008070709
Regeneration of glomerular podocytes by human renal progenitors
Elisa Ronconi et al. J Am Soc Nephrol. 2009 Feb.
Abstract
Depletion of podocytes, common to glomerular diseases in general, plays a role in the pathogenesis of glomerulosclerosis. Whether podocyte injury in adulthood can be repaired has not been established. Here, we demonstrate that in the adult human kidney, CD133+CD24+ cells consist of a hierarchical population of progenitors that are arranged in a precise sequence within Bowman's capsule and exhibit heterogeneous potential for differentiation and regeneration. Cells localized to the urinary pole that expressed CD133 and CD24, but not podocyte markers (CD133+CD24+PDX- cells), could regenerate both tubular cells and podocytes. In contrast, cells localized between the urinary pole and vascular pole that expressed both progenitor and podocytes markers (CD133+CD24+PDX+) could regenerate only podocytes. Finally, cells localized to the vascular pole did not exhibit progenitor markers, but displayed phenotypic features of differentiated podocytes (CD133-CD24-PDX+ cells). Injection of CD133+CD24+PDX- cells, but not CD133+CD24+PDX+ or CD133-CD24- cells, into mice with adriamycin-induced nephropathy reduced proteinuria and improved chronic glomerular damage, suggesting that CD133+CD24+PDX- cells could potentially treat glomerular disorders characterized by podocyte injury, proteinuria, and progressive glomerulosclerosis.
Figures
Figure 1.
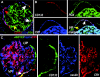
Heterogeneous expression of renal progenitors and podocytes markers by cells of Bowman's capsule in adult human kidney. (A) Triple-label immunofluorescence for CD133 (red), CD24 (blue), and PDX (green) showing co-expression of CD133 and CD24 on one subset (red arrow) and co-expression of CD133, CD24, and PDX on another subset of parietal epithelial cells in Bowman's capsule of a human kidney (white arrows). CD133−CD24−PDX+ cells are also visible (yellow arrow). Sectioning of the glomerulus does not allow its polarity to be established. Objective 20×, zoom 1.7. (B) Triple-label immunofluorescence for CD133, PDX, and CR1 showing a subset of CD133+ cells that co-express PDX and CR1 (white arrow), and more differentiated cells that co-express PDX and CR1 but lack CD133 (yellow arrow) and localize at the vascular pole (VP). Objective 20×, zoom 2.4. (C) Triple-label immunofluorescence for CD133 (green), nestin (blue), and CR1 (red) demonstrating the existence of cells expressing CD133 in absence of the podocyte markers nestin and CR1, which localize to the urinary pole (UP) of Bowman's capsule, and cells expressing CD133, nestin, and CR1 (white arrow), which localize between the urinary and the vascular pole (VP) of Bowman's capsule. Glomerular podocytes also appear as CD133−nestin+CR1+. Objective 20×, zoom 2.8.
Figure 2.
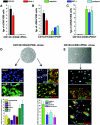
Identification of CD133+CD24+PDX−, CD133+CD24+PDX+, and CD133−CD24−PDX+ cells in adult human kidney cell suspensions. (A) After depletion of CD45+ cells, total renal cells were analyzed for the contemporaneous expression of CD133, CD24, PDX, and CD31 by triple-label immunofluorescence using FACS analysis, demonstrating that CD133+CD24+ cells represent 3.7%, whereas CD133+CD24+PDX+ cells represent 0.6 to 0.7% of total renal cells. Neither CD133+CD24+ cells nor CD133+CD24+PDX+ cells express the endothelial cell marker CD31. (B) Expression of CD133, CD24, CD31, and WT-1, as assessed by flow cytometry on PDX+ cells (left) and on PDX− cells (right). Purification of PDX+ cells confirms the existence of a population of PDX+ cells that co-express CD133 and CD24 but do not express CD31 and represent a subset of WT-1-expressing podocytes.
Figure 3.
Phenotypic characterization and distinct differentiative properties of clonal progenies of CD133+CD24+PDX− and CD133+CD24+PDX+ cells. Assessment of mRNA levels for CD133, Bmi-1, nephrin, WT-1, and podocin by real-time quantitative RT-PCR on (A) freshly isolated CD133+CD24+PDX− cells, (B) freshly isolated CD133+CD24+PDX+ cells, and (C) freshly isolated CD133−CD24−PDX+ cells. Data are mean ± SEM of triplicate assessment of one representative from independent experiments performed on sorted cells obtained from four different donors; fgs = femtograms. (D) Representative micrograph of clonally expanded CD133+CD24+PDX− cells, objective 10×. Left: Expression of tubular markers before (day 0) and after (day 21) culture in tubular differentiation medium was assessed by confocal microscopy analysis for LTA and by real-time quantitative RT-PCR comparison of multiple tubular specific markers before and after differentiation. Data are mean ± SEM of values obtained in 20 different clones. TO-PRO-3 counterstains nuclei (blue). Objective 20×, zoom 1.8. Right: Expression of podocyte markers before (day 0) and after (day 7) culture of CD133+CD24+PDX− clones in VRAD medium was assessed by confocal microscopy for CD133 (green), synaptopodin (red), nephrin (green), GLEPP (red), and by real-time quantitative RT-PCR comparison of nephrin, WT-1, podocin, and PDX mRNA levels before and after differentiation. TO-PRO-3 counterstains nuclei (blue). Objective 20×, zoom 0.9. (E) Representative micrograph of CD133+CD24+PDX+ cells (objective 20×) and staining for CD133 (green), synaptopodin (red), WT-1 (green), nephrin (green), and GLEPP-1 (red) as assessed by confocal microscopy. One representative of 20 distinct clones analyzed is shown. TO-PRO-3 counterstains nuclei (blue). Objective 20×, zoom 0.9.
Figure 4.
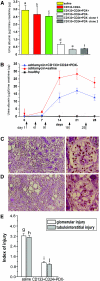
CD133+CD24+PDX− cells, but not CD133+CD24+PDX+ or CD133-CD24− cells, reduce proteinuria and improve glomerular and tubulointerstitial injury in SCID mice affected by adriamycin-induced nephropathy. (A) Albumin/creatinine ratio as measured at day 7 in adriamycin-treated mice that received saline (n = 12), CD133−CD24− (n = 6), CD133+CD24+PDX+ (n = 6), and CD133+CD24+PDX− (n = 12) bulk of cells or two representative clones of CD133+CD24+PDX− cells (n = 3 for each clone). Data are expressed as mean ± SEM. a versus b, a versus c, b versus c, d versus e, d versus f, e versus f, NS; a versus d, b versus d, c versus d, P < 0.001; a versus e, a versus f, P < 0.01; b versus e, b versus f, c versus e, c versus f, P < 0.05. (B) Time course experiments of albumin/creatinine ratio as measured in healthy (black square) or adriamycin-treated mice that received saline (red circle) or CD133+CD24+PDX− cells (blue triangle). Red arrow points to the day of adriamycin injection. Black arrows point to the days of CD133+CD24+PDX− cell injection. Data are expressed as mean ± SEM. (n = 12 mice at each time point for each group of treatment). P < 0.0001 between the two groups of treatment (ANOVA for multiple comparisons). (C) Left: PAS staining of renal cortical sections of mice with adriamycin-induced nephropathy treated with CD133+CD24+PDX− cells; objective 20×. Right: High-power magnification of a glomerulus; objective 40×. (D) Left: PAS staining of renal cortical sections of mice with adriamycin-induced nephropathy treated with saline.; objective 20×. Right: High-power magnification of a glomerulus; objective 40×. (E) Quantitation of glomerular and tubulointerstitial injuries in renal cortical sections of saline-treated and CD133+CD24+PDX− treated mice after the induction of adriamycin nephropathy. Data are expressed as mean ± SEM. g versus i and h versus j, P < 0.001.
Figure 5.
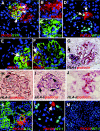
Distinct regenerative potential of CD133+CD24+PDX−, CD133+CD24+PDX+, or CD133−CD24− cells for podocytes and tubular cells in SCID mice affected by adriamycin-induced nephropathy. (A) Representative micrograph of kidney sections of mice with adriamycin-induced nephropathy treated with PKH26-labeled CD133+CD24+PDX− cells (red) and stained with synaptopodin (SYN, green), which demonstrates engraftment of CD133+CD24+PDX− cells in both glomerular and tubular structures and differentiation toward podocytes and tubular cells at day 7. TO-PRO-3 counterstains nuclei (blue). Objective 40×, zoom 1.4. (B) High magnification of the split image showed in panel a detailing PKH26 labeling in glomerular cells. TO-PRO-3 counterstains nuclei (blue). Objective 40×, zoom 1.7. (C) High magnification of the split image showed in panel a, detailing synaptopodyn (SYN) labeling in glomerular cells. TO-PRO-3 counterstains nuclei (blue). Objective 40×, zoom 1.7. (D) Representative micrograph of kidney sections of mice with adriamycin-induced nephropathy treated with PKH26-labeled CD133+CD24+PDX− cells (red) and stained with WT-1 (green), which demonstrates engraftment of CD133+CD24+PDX− cells and tubular structures and differentiation toward podocytes (arrow) and tubular cells at day 28. TO-PRO-3 counterstains nuclei (blue). Objective 40×, zoom 1.2. (E) Representative micrograph of kidney sections of mice with adriamycin-induced nephropathy treated with PKH26-labeled CD133+CD24+PDX− cells (red) and stained with nephrin (green), which demonstrates engraftment of CD133+CD24+PDX− cells in glomerular structures and differentiation toward podocytes (arrow) at day 28. TO-PRO-3 counterstains nuclei (blue). Objective 40×, zoom 1.7. (F) Representative micrograph of kidney sections of mice with adriamycin-induced nephropathy treated with PKH26-labeled CD133+CD24+PDX− cells (red) and stained with podocin (green), which demonstrates engraftment of CD133+CD24+PDX− cells in glomerular structures and differentiation toward podocytes (arrow) at day 28. TO-PRO-3 counterstains nuclei (blue). Objective 40×, zoom 1.8. (G) Double-label immunohistochemistry for podocin (AEC, red) and HLA-I human antigen (vector SG, dark blue) in a section adjacent to panel f from kidneys of SCID mice with adriamycin-induced nephropathy treated with CD133+CD24+PDX− cells, which confirms that the PKH26-labeled cells shown in panel f also exhibit HLA-I immunostaining (arrow) at day 28. Objective 40×. (H) Double-label immunohistochemistry for podocin (AEC, red) and HLA-I human antigen (vector SG, dark blue) in kidneys of SCID mice with adriamycin-induced nephropathy treated with CD133+CD24+PDX− cells, which demonstrates engraftment of a CD133+CD24+PDX− cell in a glomerulus and its differentiation toward a podocyte (arrow) at day 28. Objective 40×. (I) Double-label immunohistochemistry for podocin (AEC, red) and HLA-I human antigen (vector SG, dark blue) in kidneys of SCID mice with adriamycin-induced nephropathy treated with CD133+CD24+PDX− cells, which demonstrates engraftment of several CD133+CD24+PDX− cells and their differentiation toward podocytes (arrows) at day 28. Objective 40×. (J) Double-label immunohistochemistry for podocin (AEC, red) and HLA-I human antigen (vector SG, dark blue) in kidneys of SCID mice with adriamycin-induced nephropathy treated with saline, which shows absence of HLA-I immunostaining at day 28. Objective 40×. (K) Representative micrograph of kidney sections of mice with adriamycin-induced nephropathy treated with PKH26-labeled CD133+CD24+PDX− cells (red) and stained with LTA (green), which demonstrates engraftment of CD133+CD24+PDX− cells in proximal tubular structures and differentiation toward tubular cells at day 28. TO-PRO-3 counterstains nuclei (blue). Objective 40×. (L) Representative micrograph of kidney sections of mice with adriamycin-induced nephropathy treated with PKH26-labeled CD133+CD24+PDX+ cells (red) and stained with WT-1 (green), which demonstrates rare CD133+CD24+PDX+ cells in glomerular structures at day 28. TO-PRO-3 counterstains nuclei (blue). Objective 40×, zoom 1.7. (M) Representative micrograph of kidney sections of mice with adriamycin-induced nephropathy treated with PKH26-labeled CD133−CD24− cells (red) and stained with WT-1 (green), which demonstrates the absence of CD133−CD24− cells in glomerular or tubular structures at day 28. TO-PRO-3 counterstains nuclei (blue). Objective 20×.
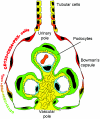
Figure 6.
Schematic representation of the hierarchical distribution of CD133+CD24+PDX− and CD133+CD24+PDX+ cells within human glomeruli. CD133+CD24+PDX− renal progenitors (red) are localized at the urinary pole in close contiguity with tubular renal cells (yellow). A transitional cell population (CD133+CD24+PDX+, red/green) displays features of either renal progenitors (red) or podocytes (green) and localizes between the urinary pole and the vascular pole. At the vascular pole of the glomerulus, the transitional cells are localized in close continuity with cells that lack CD133 and CD24, but exhibit the podocyte markers and the phenotypic features of differentiated podocytes (green).
Comment in
- Parietal epithelial cells regenerate podocytes.
Poulsom R, Little MH. Poulsom R, et al. J Am Soc Nephrol. 2009 Feb;20(2):231-3. doi: 10.1681/ASN.2008121279. Epub 2009 Jan 28. J Am Soc Nephrol. 2009. PMID: 19176695 No abstract available.
Similar articles
- Renal progenitor cells contribute to hyperplastic lesions of podocytopathies and crescentic glomerulonephritis.
Smeets B, Angelotti ML, Rizzo P, Dijkman H, Lazzeri E, Mooren F, Ballerini L, Parente E, Sagrinati C, Mazzinghi B, Ronconi E, Becherucci F, Benigni A, Steenbergen E, Lasagni L, Remuzzi G, Wetzels J, Romagnani P. Smeets B, et al. J Am Soc Nephrol. 2009 Dec;20(12):2593-603. doi: 10.1681/ASN.2009020132. Epub 2009 Oct 29. J Am Soc Nephrol. 2009. PMID: 19875807 Free PMC article. - Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury.
Angelotti ML, Ronconi E, Ballerini L, Peired A, Mazzinghi B, Sagrinati C, Parente E, Gacci M, Carini M, Rotondi M, Fogo AB, Lazzeri E, Lasagni L, Romagnani P. Angelotti ML, et al. Stem Cells. 2012 Aug;30(8):1714-25. doi: 10.1002/stem.1130. Stem Cells. 2012. PMID: 22628275 - Parietal epithelial cells regenerate podocytes.
Poulsom R, Little MH. Poulsom R, et al. J Am Soc Nephrol. 2009 Feb;20(2):231-3. doi: 10.1681/ASN.2008121279. Epub 2009 Jan 28. J Am Soc Nephrol. 2009. PMID: 19176695 No abstract available. - Toward the identification of a "renopoietic system"?
Romagnani P. Romagnani P. Stem Cells. 2009 Sep;27(9):2247-53. doi: 10.1002/stem.140. Stem Cells. 2009. PMID: 19739254 Free PMC article. Review. - The glomerulus--a view from the outside--the podocyte.
Cheng H, Harris RC. Cheng H, et al. Int J Biochem Cell Biol. 2010 Sep;42(9):1380-7. doi: 10.1016/j.biocel.2010.05.014. Epub 2010 Jun 11. Int J Biochem Cell Biol. 2010. PMID: 20542138 Free PMC article. Review.
Cited by
- C-kit(+) cells isolated from developing kidneys are a novel population of stem cells with regenerative potential.
Rangel EB, Gomes SA, Dulce RA, Premer C, Rodrigues CO, Kanashiro-Takeuchi RM, Oskouei B, Carvalho DA, Ruiz P, Reiser J, Hare JM. Rangel EB, et al. Stem Cells. 2013 Aug;31(8):1644-56. doi: 10.1002/stem.1412. Stem Cells. 2013. PMID: 23733311 Free PMC article. - The isolation and characterization of renal cancer initiating cells from human Wilms' tumour xenografts unveils new therapeutic targets.
Pode-Shakked N, Shukrun R, Mark-Danieli M, Tsvetkov P, Bahar S, Pri-Chen S, Goldstein RS, Rom-Gross E, Mor Y, Fridman E, Meir K, Simon A, Magister M, Kaminski N, Goldmacher VS, Harari-Steinberg O, Dekel B. Pode-Shakked N, et al. EMBO Mol Med. 2013 Jan;5(1):18-37. doi: 10.1002/emmm.201201516. Epub 2012 Dec 13. EMBO Mol Med. 2013. PMID: 23239665 Free PMC article. - Podocytes proliferate: novel mechanism identified in collapsing glomerulopathies.
Zhu C, Mertens PR. Zhu C, et al. Int Urol Nephrol. 2013 Feb;45(1):275-9. doi: 10.1007/s11255-012-0318-6. Epub 2012 Nov 8. Int Urol Nephrol. 2013. PMID: 23136030 Review. No abstract available. - Predicting proximal tubule failed repair drivers through regularized regression analysis of single cell multiomic sequencing.
Ledru N, Wilson PC, Muto Y, Yoshimura Y, Wu H, Li D, Asthana A, Tullius SG, Waikar SS, Orlando G, Humphreys BD. Ledru N, et al. Nat Commun. 2024 Feb 12;15(1):1291. doi: 10.1038/s41467-024-45706-0. Nat Commun. 2024. PMID: 38347009 Free PMC article. - Modeled microgravity unravels the roles of mechanical forces in renal progenitor cell physiology.
Melica ME, Cialdai F, La Regina G, Risaliti C, Dafichi T, Peired AJ, Romagnani P, Monici M, Lasagni L. Melica ME, et al. Stem Cell Res Ther. 2024 Jan 17;15(1):20. doi: 10.1186/s13287-024-03633-3. Stem Cell Res Ther. 2024. PMID: 38233961 Free PMC article.
References
- Wiggins RC: The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 - PubMed
- D'Agati VD: Podocyte injury in focal segmental glomerulosclerosis: Lessons from animal models (a play in five acts). Kidney Int 73: 399–406, 2008 - PubMed
- Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Hozman LB, Wiggins RC: Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 - PubMed
- Shankland SJ: The podocyte's response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 - PubMed
- Pavenstädt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials