Fate of culture-expanded mesenchymal stem cells in the microvasculature: in vivo observations of cell kinetics - PubMed (original) (raw)
Fate of culture-expanded mesenchymal stem cells in the microvasculature: in vivo observations of cell kinetics
Catalin Toma et al. Circ Res. 2009.
Abstract
Vascular delivery of mesenchymal stem cells (MSCs) following myocardial infarction is under clinical investigation. Little is known about the microvascular fate of MSCs. We used intravital microscopy of rat cremaster muscle microcirculation to track intraarterially delivered MSCs. Rat MSCs (average diameter, 23 microm) were bolused into the ipsilateral common iliac artery. Interrogation of an arteriole-venule pair revealed that 92+/-7% (n=6) of MSCs arrest and interrupt flow during first pass at the precapillary level, resulting in decreased flow in the feeding arteriole (velocity decreased from 6.3+/-1.0 to 4.6+/-1.3 mm/sec; P<0.001). MSC deformability evaluated using filtration through polycarbonate membranes revealed that the cortical tension of MSCs (0.49+/-0.07 dyne/cm, n=9) was not different from that of circulating mononuclear cells (0.50+/-0.05 dyne/cm, n=7). When intravital microscopy was performed 3 days following injection, the number of MSCs in the cremaster further decreased to 14% of the initial number, because of cell death in situ. In vivo labeling of the basement membrane revealed that at 1 day, the surviving cells were spread out on the luminal side of the microvessel, whereas at 3 days, they integrated in the microvascular wall. Despite their deformability, intraarterially delivered MSCs entrap at the precapillary level because of their large size, with a small proportion of surviving MSCs integrating in a perivascular niche.
Figures
Figure 1
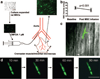
Rheological effects of intraarterially delivered MSCs. a, MSCs fluorescently labeled with CMFDA were injected above the origin of the cremaster artery, leading to diffuse precapillary cell entrapment. b, Microvascular plugging significantly decreased the RBC velocity in the feeding arteriole. c, High magnification of MSCs arrested in a microvessel with interruption of flow (arrow indicating the flow divergence point; see supplemental Video 1). d, Three entrapped MSCs observed for up to 90 minutes: no morphological changes suggestive of extravascular migration. Scale bar = 100 µm.
Figure 2
Physical properties of MSCs. a and b, Ramp–filtration curves from experiments to measure deformability of MNCs and MSCs, respectively. c, Cortical tensions (stiffness) of MSCs and circulating MNCs were comparable; after disrupting the MSC cytoskeleton with cytochalasin B (cytoB), MSC cortical tension decreased. After oxidative stress (H2O2), cell stiffness nonsignificantly increased.
Figure 3
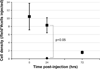
Time course of MSC persistence in cremaster microcirculation. The density of fluorescent cells (numbers of cells/field normalized to the number of injected cells in millions) decreased significantly over time (■); when MSCs underwent oxidative stress (H2O2) to induce apoptosis, virtually no cells were identified at 24 hours (◆) (n = 3, P<0.05), indicating that the remaining fluorescent cells were MSCs and not an artifact from dye transfer.
Figure 4
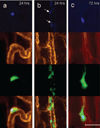
Initial fate of entrapped cells. Multicolor in vivo fluorescence imaging of MSCs in cremaster muscle. The CMFDA-labeled MSCs are green fluorescent (third row), whereas the microvascular basement membrane was stained red with rhodamine-labeled BSL I (second row). The MSCs were double-labeled with DAPI for nuclear staining (blue, top row). The bottom rows represent fused images. a, At 24 hours, the MSCs were spread out on the luminal side of the microvessels with preserved cytoplasmic and nuclear morphology. b, Heavily fragmented cells at 24 hours, with nuclear condensation (arrows) suggestive of apoptosis. c, At 72 hours, most persisting cells were morphologically intact, with preserved nuclear morphology. Scale bar = 50 µm.
Figure 5
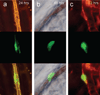
MSC localization relative to the vessel wall. Multicolor in vivo fluorescence imaging of MSCs and basement membrane in the cremaster muscle, as in Figure 4. At 24 to 48 hours, the cells were spread out on the luminal side (a and b), with restoration of flow (depicted by the column of blood in the middle of column b, representing DIC imaging at 48 hours). At 72 hours, some of the cells appear perivascular, with presence of a basement membrane between the MSC and the microvessel (c). Scale bar = 50 µm.
Figure 6
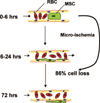
Hypothesis for the intravascular fate of culture-expanded MSCs. After intraarterial MSC injection, microvascular plugging with obstruction of flow occurs at the precapillary level. The resulting ischemia leads to loss of most of the injected cells (86%). The few surviving cells initially spread out on the luminal side of the vessel and then localize in a perivascular niche.
Comment in
- Where have all the stem cells gone?
Bagi Z, Kaley G. Bagi Z, et al. Circ Res. 2009 Feb 13;104(3):280-1. doi: 10.1161/CIRCRESAHA.108.192641. Circ Res. 2009. PMID: 19213961 Free PMC article. No abstract available.
Similar articles
- Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy.
Furlani D, Ugurlucan M, Ong L, Bieback K, Pittermann E, Westien I, Wang W, Yerebakan C, Li W, Gaebel R, Li RK, Vollmar B, Steinhoff G, Ma N. Furlani D, et al. Microvasc Res. 2009 May;77(3):370-6. doi: 10.1016/j.mvr.2009.02.001. Epub 2009 Feb 26. Microvasc Res. 2009. PMID: 19249320 - Mesenchymal Stem Cell Deformability and Implications for Microvascular Sequestration.
Lipowsky HH, Bowers DT, Banik BL, Brown JL. Lipowsky HH, et al. Ann Biomed Eng. 2018 Apr;46(4):640-654. doi: 10.1007/s10439-018-1985-y. Epub 2018 Jan 19. Ann Biomed Eng. 2018. PMID: 29352448 Free PMC article. - In vivo efficacy of endothelial growth medium stimulated mesenchymal stem cells derived from patients with critical limb ischemia.
Al-Rifai R, Nguyen P, Bouland N, Terryn C, Kanagaratnam L, Poitevin G, François C, Boisson-Vidal C, Sevestre MA, Tournois C. Al-Rifai R, et al. J Transl Med. 2019 Aug 9;17(1):261. doi: 10.1186/s12967-019-2003-3. J Transl Med. 2019. PMID: 31399109 Free PMC article. - Mesenchymal stem cells injected into carotid artery to target focal brain injury home to perivascular space.
Andrzejewska A, Dabrowska S, Nowak B, Walczak P, Lukomska B, Janowski M. Andrzejewska A, et al. Theranostics. 2020 May 17;10(15):6615-6628. doi: 10.7150/thno.43169. eCollection 2020. Theranostics. 2020. PMID: 32550893 Free PMC article. - Different cardiovascular potential of adult- and fetal-type mesenchymal stem cells in a rat model of heart cryoinjury.
Iop L, Chiavegato A, Callegari A, Bollini S, Piccoli M, Pozzobon M, Rossi CA, Calamelli S, Chiavegato D, Gerosa G, De Coppi P, Sartore S. Iop L, et al. Cell Transplant. 2008;17(6):679-94. doi: 10.3727/096368908786092739. Cell Transplant. 2008. PMID: 18819256
Cited by
- Shattering barriers toward clinically meaningful MSC therapies.
Levy O, Kuai R, Siren EMJ, Bhere D, Milton Y, Nissar N, De Biasio M, Heinelt M, Reeve B, Abdi R, Alturki M, Fallatah M, Almalik A, Alhasan AH, Shah K, Karp JM. Levy O, et al. Sci Adv. 2020 Jul 22;6(30):eaba6884. doi: 10.1126/sciadv.aba6884. eCollection 2020 Jul. Sci Adv. 2020. PMID: 32832666 Free PMC article. Review. - Pretreatment of Mesenchymal Stem Cells Manipulates Their Vasculoprotective Potential While Not Altering Their Homing Within the Injured Gut.
Kavanagh DP, Suresh S, Newsome PN, Frampton J, Kalia N. Kavanagh DP, et al. Stem Cells. 2015 Sep;33(9):2785-97. doi: 10.1002/stem.2061. Epub 2015 Jun 29. Stem Cells. 2015. PMID: 26124062 Free PMC article. - Protective effects of mesenchymal stromal cell-derived secretome on dermonecrosis induced in rabbits by Loxosceles intermedia spider venom.
Rodrigues GM, de Almeida ME, Marcelino SAC, Fernandes PBU, da Cruz JOP, Araújo FL, Ferreira RDS, Botelho AFM, Bedoya FJ, Cahuana GM, Hitos AB, Soria B, Costal-Oliveira F, Duarte CG, Tejedo JR, Chávez-Olórtegui C, Melo MM. Rodrigues GM, et al. J Venom Anim Toxins Incl Trop Dis. 2024 Jul 22;30:e20240004. doi: 10.1590/1678-9199-JVATITD-2024-0004. eCollection 2024. J Venom Anim Toxins Incl Trop Dis. 2024. PMID: 39069986 Free PMC article. - Multipotent mesenchymal stromal cells and the innate immune system.
Le Blanc K, Mougiakakos D. Le Blanc K, et al. Nat Rev Immunol. 2012 Apr 25;12(5):383-96. doi: 10.1038/nri3209. Nat Rev Immunol. 2012. PMID: 22531326 Review. - Optimal H2O2 preconditioning to improve bone marrow mesenchymal stem cells' engraftment in wound healing.
Guo L, Du J, Yuan DF, Zhang Y, Zhang S, Zhang HC, Mi JW, Ning YL, Chen MJ, Wen DL, Sun JH, Liu D, Zeng L, Zhang A, Jiang J, Huang H. Guo L, et al. Stem Cell Res Ther. 2020 Oct 8;11(1):434. doi: 10.1186/s13287-020-01910-5. Stem Cell Res Ther. 2020. PMID: 33032649 Free PMC article.
References
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. - PubMed
- Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. - PubMed
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
- Jones E, McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology (Oxford) 2008;47:126–131. - PubMed
- Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringdén O. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources