Induced pluripotent stem cell generation using a single lentiviral stem cell cassette - PubMed (original) (raw)
Induced pluripotent stem cell generation using a single lentiviral stem cell cassette
Cesar A Sommer et al. Stem Cells. 2009 Mar.
Abstract
Induced pluripotent stem (iPS) cells can be generated using retroviral vectors expressing Oct4, Klf4, Sox2, and cMyc. Most prior studies have required multiple retroviral vectors for reprogramming, resulting in high numbers of genomic integrations in iPS cells and limiting their use for therapeutic applications. Here we describe the use of a single lentiviral vector expressing a "stem cell cassette" composed of the four transcription factors and a combination of 2A peptide and internal ribosome entry site technology, generating iPS cells from postnatal fibroblasts. iPS cells generated in this manner display embryonic stem cell-like morphology, express stem cell markers, and exhibit in vivo pluripotency, as evidenced by their ability to differentiate in teratoma assays and their robust contribution to mouse chimeras. Combining all factors into a single transcript achieves the most efficient reprogramming system to date and allows derivation of iPS cells with a single viral integration. The use of a single lentiviral vector for reprogramming represents a powerful laboratory tool and a significant step toward the application of iPS technology for clinical purposes.
Figures
Figure 1. Generation of a single lentiviral vector expressing a stem cell cassette
(A) Schematic representation of pHAGE-STEMCCA (inducible version). The engineered stem cell cassette consists of a single multicistronic mRNA transcribed under the control of a doxycycline-inducible TetO-miniCMV promoter. The mRNA contains an IRES element separating two fusion cistrons. The two cistrons consist of Oct4 and Sox2 coding sequences fused to Klf4 and cMyc, respectively, through the use of intervening sequences encoding ‘self-cleaving’ 2A peptides (F2A and E2A). LTR: long terminal repeat; PSI: packaging signal; RRE: rev responsive element; cpPu: central polypuryne tract; WPRE: Woodchuck hepatitis virus post-transcriptional regulatory element; dU3: deleted U3. (B) Western blot analysis of lysates from 293T transfected cells. Cells were co-transfected with pHAGE-Tet-STEMCCA and a vector expressing the rtTA protein and maintained in doxycycline for 72 h before lysate preparation. Cells transfected with either mock vectors or four monocistronic pHAGE vectors encoding the four individual transcription factors were used as negative (-) or positive (+) controls, respectively. (C) Immunofluorescence microscopy of MEFs infected 4 days earlier with pHAGE-EF1α-STEMCCA shows expression of all four transcription factors. Uninfected MEFs or secondary antibody only staining control showed no detectable staining.
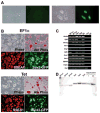
Figure 2. Characterization of iPS cells generated using a single lentiviral vector
(A) Representative pictures of TTFs from a Sox2-GFP M2rtTA mouse transduced with the inducible STEMCCA vector, in the absence (left panel) or presence (right panel) of doxycycline induction. Images were acquired on day 14 post-infection. (B) Representative pictures of iPS cells derived using the constitutive pHAGE-EF1α-STEMCCA (EF1α) or the inducible pHAGE-Tet-STEMCCA vector (Tet) show colony morphology (Phase), high alkaline phosphatase activity (Alk Phos), SSEA1 immunostaining, and Sox2-GFP reporter gene expression. (C) Expression of ESC ‘marker’ genes detected by RT-PCR in four representative iPS cell clones generated by the constitutive (EF1α) or inducible (Tet) STEMCCA vector. Nat1 is a constitutively expressed gene and serves as a control for loading. Representative samples from unmanipulated tail tip fibroblasts (TTF) and mouse ES cells are also shown. An iPS cell sample prepared without RT was used as negative control (-RT). (D) Southern blot analysis of genomic DNA (gDNA) purified from 6 representative iPS clones produced with the constitutive (EF1α) or inducible (Tet) vector. gDNA was digested with BglII to obtain a band of 6.7Kb in the EF1α colonies or 8.3Kb in the Tet colonies, representing most of the proviral genome. For control, pHAGE-Tet-STEMCCA plasmid DNA representing 1 or 2.5 copies of the insert was digested with BglII. A single band of the expected size of the proviral gene insertion is present in all clones. The density of each band indicates between 1–3 proviral integrations in each clone.
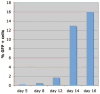
Figure 3. Dynamics of reprogramming using a single lentiviral stem cell cassette
Analysis of GFP expression over time in TTFs purified from Sox2-GFP M2rtTA double knock-in mice infected with pHAGE-Tet-STEMCCA vector. Transduced cells from independent wells were collected at each time point following doxycycline exposure. GFP expression was analyzed using a FACScan machine. The Day 5 result was indistinguishable from background GFP expression.
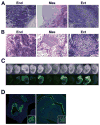
Figure 4. iPS cells generated with a single lentiviral vector are pluripotent
(A and B) Teratomas derived from iPS cell lines produced with pHAGE-EF1α-STEMCCA (A) or pHAGE-Tet-STEMCCA (B) vector, showing differentiation into cell types of all three germ layers: endoderm (end), mesoderm (mes) and ectoderm (ect). Images are representative of two independent experiments testing three individual iPS clones for each construct. (C) iPS cells generated with pHAGE-Tet-STEMCCA vector from TTFs of a Sox2-GFP Rosa26-M2rtTA mouse show high levels of embryonic contribution following injections into blastocysts. Chimerism is evidenced by Sox2-GFP expression in neural crest-derived tissues in 9 of 12 mid-term embryos. (D) Contribution to neuroectoderm (left panel: olphactory region; inset: developing brain) and endoderm (right panel: oropharynx; inset: proximal lung epithelium) is evidenced by Sox2-GFP expression in histological sections of mid-term chimeric embryos.
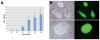
Figure 5. Reactivation of STEMCCA
(A) MEFs were obtained from E11.5 chimeric embryos generated by iPS cells derived from TTFs of Sox2-GFP mice transduced with pHAGE-Tet-STEMCCA. MEFs were exposed to doxycycline (dox) for varying times and GFP colonies were counted on day 22. Stable iPS clones expressing Sox2-GFP were derived from cells exposed to docycycline for as little as 4 days. Experiments were done in duplicates. n.d.= not detected. (B) Colony formation and Sox2-GFP expression in 2 representative fields is shown. Parallel samples cultured in the absence of dox showed no GFP expression and no morphological changes.
Similar articles
- Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells.
Zhou W, Freed CR. Zhou W, et al. Stem Cells. 2009 Nov;27(11):2667-74. doi: 10.1002/stem.201. Stem Cells. 2009. PMID: 19697349 - Polycistronic lentiviral vector for "hit and run" reprogramming of adult skin fibroblasts to induced pluripotent stem cells.
Chang CW, Lai YS, Pawlik KM, Liu K, Sun CW, Li C, Schoeb TR, Townes TM. Chang CW, et al. Stem Cells. 2009 May;27(5):1042-9. doi: 10.1002/stem.39. Stem Cells. 2009. PMID: 19415770 - Emerging methods for preparing iPS cells.
Miyazaki S, Yamamoto H, Miyoshi N, Takahashi H, Suzuki Y, Haraguchi N, Ishii H, Doki Y, Mori M. Miyazaki S, et al. Jpn J Clin Oncol. 2012 Sep;42(9):773-9. doi: 10.1093/jjco/hys108. Epub 2012 Jul 23. Jpn J Clin Oncol. 2012. PMID: 22826352 Review. - Generation of clinically relevant "induced pluripotent stem" (iPS) cells.
Heffernan C, Sumer H, Verma PJ. Heffernan C, et al. J Stem Cells. 2011;6(3):109-27. J Stem Cells. 2011. PMID: 23264997 Review.
Cited by
- A poor imitation of a natural process: a call to reconsider the iPSC engineering technique.
Zhang Y, Yao L, Yu X, Ou J, Hui N, Liu S. Zhang Y, et al. Cell Cycle. 2012 Dec 15;11(24):4536-44. doi: 10.4161/cc.22575. Epub 2012 Oct 31. Cell Cycle. 2012. PMID: 23114619 Free PMC article. Review. - Gene therapy for atrial fibrillation.
Mo W, Donahue JK. Mo W, et al. J Mol Cell Cardiol. 2024 Nov;196:84-93. doi: 10.1016/j.yjmcc.2024.09.004. Epub 2024 Sep 11. J Mol Cell Cardiol. 2024. PMID: 39270930 Review. - Nuclear reprogramming with a non-integrating human RNA virus.
Driscoll CB, Tonne JM, El Khatib M, Cattaneo R, Ikeda Y, Devaux P. Driscoll CB, et al. Stem Cell Res Ther. 2015 Mar 26;6(1):48. doi: 10.1186/s13287-015-0035-z. Stem Cell Res Ther. 2015. PMID: 25889591 Free PMC article. - Development of an all-in-one inducible lentiviral vector for gene specific analysis of reprogramming.
Yamaguchi T, Hamanaka S, Kamiya A, Okabe M, Kawarai M, Wakiyama Y, Umino A, Hayama T, Sato H, Lee YS, Kato-Itoh M, Masaki H, Kobayashi T, Yamazaki S, Nakauchi H. Yamaguchi T, et al. PLoS One. 2012;7(7):e41007. doi: 10.1371/journal.pone.0041007. Epub 2012 Jul 18. PLoS One. 2012. PMID: 22815895 Free PMC article. - Induced pluripotent stem cells as a model for diabetes investigation.
Stepniewski J, Kachamakova-Trojanowska N, Ogrocki D, Szopa M, Matlok M, Beilharz M, Dyduch G, Malecki MT, Jozkowicz A, Dulak J. Stepniewski J, et al. Sci Rep. 2015 Feb 26;5:8597. doi: 10.1038/srep08597. Sci Rep. 2015. PMID: 25716801 Free PMC article.
References
- Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. - PubMed
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
- Maherali N, Sridharan R, Xie W, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. - PubMed
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. - PubMed
- Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DP2 OD003266/OD/NIH HHS/United States
- P01 HL047049/HL/NHLBI NIH HHS/United States
- R21 HD060308/HD/NICHD NIH HHS/United States
- P01-HL047049-16A1/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources