Dissecting the cell entry pathway of dengue virus by single-particle tracking in living cells - PubMed (original) (raw)
Dissecting the cell entry pathway of dengue virus by single-particle tracking in living cells
Hilde M van der Schaar et al. PLoS Pathog. 2008 Dec.
Abstract
Dengue virus (DENV) is an enveloped RNA virus that causes the most common arthropod-borne infection worldwide. The mechanism by which DENV infects the host cell remains unclear. In this work, we used live-cell imaging and single-virus tracking to investigate the cell entry, endocytic trafficking, and fusion behavior of DENV. Simultaneous tracking of DENV particles and various endocytic markers revealed that DENV enters cells exclusively via clathrin-mediated endocytosis. The virus particles move along the cell surface in a diffusive manner before being captured by a pre-existing clathrin-coated pit. Upon clathrin-mediated entry, DENV particles are transported to Rab5-positive endosomes, which subsequently mature into late endosomes through acquisition of Rab7 and loss of Rab5. Fusion of the viral membrane with the endosomal membrane was primarily detected in late endosomal compartments.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
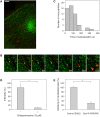
Figure 1. DENV enters cells via clathrin-mediated endocytosis.
(A) Fluorescent image of a LCa-eYFP-expressing cell (green) infected with DiD-labeled DENV particles (red). Scale bar is 10 µm. (B) Selected frames from a single DENV particle (red), indicated with the arrow, entering a cell via clathrin-mediated endocytosis. The numbers designate seconds post-binding. (C) Histogram of the time that DENV particles colocalized with clathrin-eYFP. (D) DENV infectivity in BS-C-1 cells in the absence or presence of 15 µM chlorpromazine. Viral infectivity was measured at 24 hours post-infection by counting the number of E protein–expressing cells using immunofluorescence microscopy. The experiment was performed in triplicate, and the bars represent the average±SD. * P<0.01. (E) DENV infection in HeLa cells expressing a dominant-negative Eps15 mutant (E95/295) or its vector control (D3Δ2). At 30 hours post-transfection, HeLa cells were infected with DENV for 21 hours and subsequently stained for expression of E-proteins. Cells were analyzed by flow cytometry, and the results are expressed as the percentage infectivity in transfected cells. The experiment was performed in triplicate, and the bars represent the average±SD. * P<0.01.
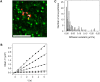
Figure 2. DENV diffuses along the cell surface and joins a pre-existing clathrin-coated pit.
(A) Overlay of a series of time-lapsed images of a virus particle prior to entering a pre-existing clathrin-coated pit. Each depicted virus image (red) is taken 4 seconds apart, and the positions are connected in time with white arrows. For clarity, the LCa-eYFP signal (green) was averaged over 10 seconds, starting at the moment when the virus first overlaps the clathrin-coated pit. Scale bar is 10 µm. (B) MSD-plot for 5 example virus trajectories prior to association with clathrin-coated pits. The diffusion constants are 0.003 µm2/s (squares), 0.009 µm2/s (asterisks), 0.047 µm2/s (triangles), 0.107 µm2/s (diamonds), and 0.159 µm2/s (circles). (C) Diffusion constants of virus particles prior to entrance into a clathrin-coated pit (light gray bars), during colocalization with clathrin (black bars), and on cells treated with chlorpromazine (dashed bars).
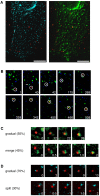
Figure 3. DENV particles are transported to early endosomes that mature into late endosomes.
(A) Fluorescent images of a cell expressing Rab5-eCFP (light blue) and Rab7-eYFP (green) upon infection with DiD-labeled DENV (red). Scale bar is 10 µm. (B) The selected frames show the endocytic trafficking behavior of a single DiD-labeled DENV particle (surrounded by a white circle) in a cell. The numbers designate seconds post-infection. (C) Snapshots of a virus trajectory showing the modes of Rab7 accumulation. At all time frames, the virus particle colocalizes with Rab5, but for clarity the signal is not depicted. Two modes of Rab7 accumulation were observed: a gradual recruitment of Rab7 (upper panel) or merging of the endosome with an existing Rab7-positive endosome (lower panel) was observed. The numbers indicate the time frame (in seconds) of endosome maturation. (D) Snapshots of a virus showing the modes of Rab5 exit. At all time frames, the virus particle colocalizes with Rab7, but for clarity the signal is not depicted. The exit of Rab5 signal also appears to take place in two different modes: a gradual release of Rab5 (upper panel) or splitting off a Rab5-containing endosome (lower panel).
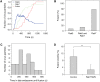
Figure 4. DENV fuses primarily from within late endosomes.
(A) Individual DENV trajectories were aligned to the time of binding to the cell surface. Fraction of virus particles that colocalize with Rab5 and Rab7, and that undergo membrane fusion are shown as a function of time. (B) Quantification of the percentage of membrane fusion events in the different endocytic compartments. (C) Histogram of the time that virus particles spend in Rab7-positive late endosomes prior to the onset of membrane fusion. (D) The number of DENV membrane fusion events in BS-C-1 cells transfected with Rab7T22N or with a control plasmid. The experiment was carried out more than 10 times and the bars represent the mean±SD. ** P<0.001.
Similar articles
- Small interference RNA profiling reveals the essential role of human membrane trafficking genes in mediating the infectious entry of dengue virus.
Ang F, Wong AP, Ng MM, Chu JJ. Ang F, et al. Virol J. 2010 Feb 1;7:24. doi: 10.1186/1743-422X-7-24. Virol J. 2010. PMID: 20122152 Free PMC article. - Differential requirements in endocytic trafficking for penetration of dengue virus.
Acosta EG, Castilla V, Damonte EB. Acosta EG, et al. PLoS One. 2012;7(9):e44835. doi: 10.1371/journal.pone.0044835. Epub 2012 Sep 7. PLoS One. 2012. PMID: 22970315 Free PMC article. - Functional entry of dengue virus into Aedes albopictus mosquito cells is dependent on clathrin-mediated endocytosis.
Acosta EG, Castilla V, Damonte EB. Acosta EG, et al. J Gen Virol. 2008 Feb;89(Pt 2):474-484. doi: 10.1099/vir.0.83357-0. J Gen Virol. 2008. PMID: 18198378 - Role of angiotensin II in cellular entry and replication of dengue virus.
Pedreañez A, Carrero Y, Vargas R, Hernández-Fonseca JP, Mosquera JA. Pedreañez A, et al. Arch Virol. 2024 May 16;169(6):121. doi: 10.1007/s00705-024-06040-4. Arch Virol. 2024. PMID: 38753119 Review. - Early dengue virus interactions: the role of dendritic cells during infection.
Santos Souza HF, da Silva Almeida B, Boscardin SB. Santos Souza HF, et al. Virus Res. 2016 Sep 2;223:88-98. doi: 10.1016/j.virusres.2016.07.001. Epub 2016 Jul 2. Virus Res. 2016. PMID: 27381061 Review.
Cited by
- Imaging of Viral Genomic RNA Replication with Nanoprobes.
Hu PP, Zheng LL, Zhan L, Huang CZ. Hu PP, et al. Methods Mol Biol. 2025;2875:145-153. doi: 10.1007/978-1-0716-4248-1_12. Methods Mol Biol. 2025. PMID: 39535646 - The mechanisms of Zika virus-induced neuropathogenesis.
Hassaan NA, Xing L. Hassaan NA, et al. Braz J Microbiol. 2024 Oct 18. doi: 10.1007/s42770-024-01543-3. Online ahead of print. Braz J Microbiol. 2024. PMID: 39422868 Review. - A review of virus host factor discovery using CRISPR screening.
See WR, Yousefi M, Ooi YS. See WR, et al. mBio. 2024 Nov 13;15(11):e0320523. doi: 10.1128/mbio.03205-23. Epub 2024 Oct 18. mBio. 2024. PMID: 39422472 Free PMC article. Review. - Early Steps of Individual Multireceptor Viral Interactions Dissected by High-Density, Multicolor Quantum Dot Mapping in Living Cells.
Mateos N, Gutierrez-Martinez E, Angulo-Capel J, Carlon-Andres I, Padilla-Parra S, Garcia-Parajo MF, Torreno-Pina JA. Mateos N, et al. ACS Nano. 2024 Oct 22;18(42):28881-28893. doi: 10.1021/acsnano.4c09085. Epub 2024 Oct 10. ACS Nano. 2024. PMID: 39387532 Free PMC article. - Natural products and derivatives as Japanese encephalitis virus antivirals.
Mi Y, Guo Y, Luo X, Bai Y, Chen H, Wang M, Wang Y, Guo J. Mi Y, et al. Pathog Dis. 2024 Feb 7;82:ftae022. doi: 10.1093/femspd/ftae022. Pathog Dis. 2024. PMID: 39317665 Free PMC article. Review.
References
- Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10:100–103. - PubMed
- Guzman MG, Kouri G. Dengue: An update. Lancet Infect Dis. 2002;2:33–42. - PubMed
- Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: The spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10:S98–S109. - PubMed
- Lindenbach D, Rice CM. Flaviviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 991–1041.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources