Attenuation of basal and cocaine-enhanced locomotion and nucleus accumbens dopamine in cannabinoid CB1-receptor-knockout mice - PubMed (original) (raw)
Attenuation of basal and cocaine-enhanced locomotion and nucleus accumbens dopamine in cannabinoid CB1-receptor-knockout mice
Xia Li et al. Psychopharmacology (Berl). 2009 May.
Abstract
Rationale: Effect of cannabinoid CB1 receptor deletion on cocaine's actions is controversial. This is partly based on findings in CB1-receptor-knockout (CB1(-/-)) mice with CD1 genetic background.
Objectives: In the present study, we used CB1(-/-) mice with a C57BL/6J genetic background to further investigate the role of CB1 receptors in cocaine's action.
Materials and methods: Locomotor activity was assessed using AccuScan locomotor chambers. Brain extracellular dopamine (DA) levels were measured by in vivo microdialysis and by fast-scan cyclic voltammetry in the nucleus accumbens (NAc).
Results: CB1(-/-) mice displayed a significant reduction in basal levels of locomotion and extracellular DA, as well as in cocaine-enhanced locomotion and extracellular DA, as compared to their wild-type (CB1(+/+)) littermates. The reduction in basal and cocaine-enhanced DA appears to be related to a reduction in basal DA release, not to an increase in DA clearance, as indicated by fast-scan cyclic voltammetry in brain slices. Pharmacological blockade of CB1 receptors by SR141716 inhibited locomotion and NAc DA release in CB1(+/+) mice.
Conclusions: The present findings suggest an important role for CB1 receptors in mediating cocaine's behavioral and neurochemical effects.
Figures
Fig. 1
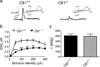
Basal and cocaine-enhanced locomotor activity in CB1+/+ and CB1−/− mice. a shows basal locomotion data (distance) and cocaine-or saline-induced locomotion in CB1+/+ and CB1−/− mice. b shows mean basal locomotion in CB1+/+ and CB1−/− mice, indicating a significant reduction in locomotion in CB1−/− mice relative to their wild-type CB1+/+ littermates. c shows percent change from baseline in cocaine-induced locomotion, indicating that the duration of cocaine-enhanced locomotion was shorter in CB1−/− mice than in CB1+/+ mice. *p<0.05, **p<0.01, ***p<0.001, compared to CB1+/+ mice. ##p<0.01, ###p<0.001, compared to baseline
Fig. 2
Basal and cocaine-enhanced NAc DA in CB1+/+ and CB1−/− mice. a shows basal and cocaine- or saline-induced changes in NAc DA in CB1+/+ and CB1−/− mice. b shows mean basal extracellular DA, indicating a significant attenuation of basal DA in CB1−/− mice relative to wild-type CB1+/+ littermates. c shows percent change in DA from baseline, indicating a significant attenuation of cocaine-enhanced DA in CB1−/− mice relative to CB1+/+ mice. *p<0.05, **p< 0.01, ***p<0.001, compared to CB1+/+ mice. #p<0.05, ##p<0.01, ###p<0.001, compared to baseline
Fig. 3
NAc DA release in CB1−/− mice. a shows representative voltammetric signals recorded in brain slices from a CB1+/+ mouse and a CB1−/− mouse. A single-pulse stimulus (1 ms, 100 µA; arrowhead) was used to elicit NAc DA release. Inset of each signal shows the background-subtracted voltammogram obtained at the peak of the recorded current. b shows the input–output relationship of DA release elicited by single-pulse stimulation in the NAc, indicating attenuated DA release across a range of stimulus intensities in CB1−/− mice (_n_=28 slices from eight subjects) relative to CB1+/+ mice (_n_=23 slices from six subjects). c shows the mean decay time constants (tau, τ) of the DA signals, indicating that DA clearance (or uptake) was not different between CB1+/+ (_n_=18 slices from six subjects) and CB1−/− mice (_n_=15 slices from four subjects). **p<0.01, ***p<0.001, compared to CB1+/+ mice, repeated-measures ANOVA
Fig. 4
Effect of SR141716 on locomotor activity in CB1+/+ mice. a shows locomotion after SR141716 (3, 10 mg/kg, i.p.) or vehicle. b shows overall mean locomotion (AUC data) after SR141716 or vehicle administration. ***p<0.001, compared to vehicle group
Fig. 5
Effect of SR141716 on extracellular NAc DA levels in CB1+/+ mice. a shows extracellular DA after SR141716 (3 mg/kg, i.p.) or vehicle. b shows overall mean NAc DA (AUC data) after SR141716 or vehicle administration. **p<0.01, compared to vehicle group
Fig. 6
Microdialyses probe locations in the NAc of mice. a Shows a representative probe track. Lines in b indicate the placement of probes in both the core and the shell of the NAc
Similar articles
- Cocaine-induced endocannabinoid release modulates behavioral and neurochemical sensitization in mice.
Mereu M, Tronci V, Chun LE, Thomas AM, Green JL, Katz JL, Tanda G. Mereu M, et al. Addict Biol. 2015 Jan;20(1):91-103. doi: 10.1111/adb.12080. Epub 2013 Aug 4. Addict Biol. 2015. PMID: 23910902 Free PMC article. - Sensitization to cocaine is inhibited after intra-accumbal GR103691 or rimonabant, but it is enhanced after co-infusion indicating functional interaction between accumbens D(3) and CB1 receptors.
Ramiro-Fuentes S, Fernandez-Espejo E. Ramiro-Fuentes S, et al. Psychopharmacology (Berl). 2011 Apr;214(4):949-59. doi: 10.1007/s00213-010-2104-4. Epub 2010 Dec 3. Psychopharmacology (Berl). 2011. PMID: 21128069 - Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens.
Xi ZX, Gilbert JG, Peng XQ, Pak AC, Li X, Gardner EL. Xi ZX, et al. J Neurosci. 2006 Aug 16;26(33):8531-6. doi: 10.1523/JNEUROSCI.0726-06.2006. J Neurosci. 2006. PMID: 16914679 Free PMC article. - mGluR5 antagonism inhibits cocaine reinforcement and relapse by elevation of extracellular glutamate in the nucleus accumbens via a CB1 receptor mechanism.
Li X, Peng XQ, Jordan CJ, Li J, Bi GH, He Y, Yang HJ, Zhang HY, Gardner EL, Xi ZX. Li X, et al. Sci Rep. 2018 Feb 27;8(1):3686. doi: 10.1038/s41598-018-22087-1. Sci Rep. 2018. PMID: 29487381 Free PMC article. - Intra-accumbens rimonabant is rewarding but induces aversion to cocaine in cocaine-treated rats, as does in vivo accumbal cannabinoid CB1 receptor silencing: critical role for glutamate receptors.
Ramiro-Fuentes S, Ortiz O, Moratalla R, Fernandez-Espejo E. Ramiro-Fuentes S, et al. Neuroscience. 2010 May 5;167(2):205-15. doi: 10.1016/j.neuroscience.2010.02.019. Epub 2010 Feb 16. Neuroscience. 2010. PMID: 20167255
Cited by
- CB1 Receptor Silencing Attenuates Ketamine-Induced Hyperlocomotion Without Compromising Its Antidepressant-Like Effects.
Gobira PH, LaMar J, Marques J, Sartim A, Silveira K, Santos L, Wegener G, Guimaraes FS, Mackie K, Lu HC, Joca S. Gobira PH, et al. Cannabis Cannabinoid Res. 2023 Oct;8(5):768-778. doi: 10.1089/can.2022.0072. Epub 2022 Sep 2. Cannabis Cannabinoid Res. 2023. PMID: 36067014 Free PMC article. - Attenuation of Cocaine-Induced Conditioned Place Preference and Motor Activity via Cannabinoid CB2 Receptor Agonism and CB1 Receptor Antagonism in Rats.
Delis F, Polissidis A, Poulia N, Justinova Z, Nomikos GG, Goldberg SR, Antoniou K. Delis F, et al. Int J Neuropsychopharmacol. 2017 Mar 1;20(3):269-278. doi: 10.1093/ijnp/pyw102. Int J Neuropsychopharmacol. 2017. PMID: 27994006 Free PMC article. - To Act or Not to Act: Endocannabinoid/Dopamine Interactions in Decision-Making.
Hernandez G, Cheer JF. Hernandez G, et al. Front Behav Neurosci. 2015 Dec 17;9:336. doi: 10.3389/fnbeh.2015.00336. eCollection 2015. Front Behav Neurosci. 2015. PMID: 26733830 Free PMC article. Review. - Temporally dependent changes in cocaine-induced synaptic plasticity in the nucleus accumbens shell are reversed by D1-like dopamine receptor stimulation.
Ortinski PI, Vassoler FM, Carlson GC, Pierce RC. Ortinski PI, et al. Neuropsychopharmacology. 2012 Jun;37(7):1671-82. doi: 10.1038/npp.2012.12. Epub 2012 Mar 14. Neuropsychopharmacology. 2012. PMID: 22414814 Free PMC article. - Endocannabinoids promote cocaine-induced impulsivity and its rapid dopaminergic correlates.
Hernandez G, Oleson EB, Gentry RN, Abbas Z, Bernstein DL, Arvanitogiannis A, Cheer JF. Hernandez G, et al. Biol Psychiatry. 2014 Mar 15;75(6):487-98. doi: 10.1016/j.biopsych.2013.09.005. Epub 2013 Oct 17. Biol Psychiatry. 2014. PMID: 24138924 Free PMC article.
References
- Abel EL. Effects of the marihuana-homologue, parahexyl, on open field behaviour in the rat. J Pharm Pharmacol. 1970;22:785. - PubMed
- Ballesteros-Yáñez I, Valverde O, Ledent C, Maldonado R, DeFelipe J. Chronic cocaine treatment alters dendritic arborization in the adult motor cortex through a CB1 cannabinoid receptor-dependent mechanism. Neuroscience. 2007;146:1536–1545. - PubMed
- Cahill PS, Walker QD, Finnegan JM, Mickelson GE, Travis ER, Wightman RM. Microelectrodes for the measurement of catecholamines in biological systems. Anal Chem. 1996;68:3180–3186. - PubMed
- Caillé S, Parsons LH. Cannabinoid modulation of opiate reinforcement through the ventral striatopallidal pathway. Neuro-psychopharmacology. 2006;31:804–813. - PubMed
- Centonze D, Battista N, Rossi S, Mercuri NB, Finazzi-Agrò A, Bernardi G, Calabresi P, Maccarrone M. A critical interaction between dopamine D2 receptors and endocannabinoids mediates the effects of cocaine on striatal GABAergic transmission. Neuropsychopharmacology. 2004;29:1488–1497. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources