Identifying neural drivers with functional MRI: an electrophysiological validation - PubMed (original) (raw)
Identifying neural drivers with functional MRI: an electrophysiological validation
Olivier David et al. PLoS Biol. 2008.
Abstract
Whether functional magnetic resonance imaging (fMRI) allows the identification of neural drivers remains an open question of particular importance to refine physiological and neuropsychological models of the brain, and/or to understand neurophysiopathology. Here, in a rat model of absence epilepsy showing spontaneous spike-and-wave discharges originating from the first somatosensory cortex (S1BF), we performed simultaneous electroencephalographic (EEG) and fMRI measurements, and subsequent intracerebral EEG (iEEG) recordings in regions strongly activated in fMRI (S1BF, thalamus, and striatum). fMRI connectivity was determined from fMRI time series directly and from hidden state variables using a measure of Granger causality and Dynamic Causal Modelling that relates synaptic activity to fMRI. fMRI connectivity was compared to directed functional coupling estimated from iEEG using asymmetry in generalised synchronisation metrics. The neural driver of spike-and-wave discharges was estimated in S1BF from iEEG, and from fMRI only when hemodynamic effects were explicitly removed. Functional connectivity analysis applied directly on fMRI signals failed because hemodynamics varied between regions, rendering temporal precedence irrelevant. This paper provides the first experimental substantiation of the theoretical possibility to improve interregional coupling estimation from hidden neural states of fMRI. As such, it has important implications for future studies on brain connectivity using functional neuroimaging.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures
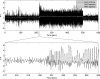
Figure 1. EEG Preprocessing
Upper panel: black (“EEG [4 20 Hz]”): 15 min of EEG recordings (band-pass filtered between 4 and 20 Hz) during EPI acquisition obtained in one rat. White (“SWD detection”): EEG power in the 4–20 Hz range (shifted to zero in between SWDs). Grey (“fMRI regressor”): previous EEG power convolved with a canonical HRF. Lower panel: short time window showing the EEG at seizure onset.
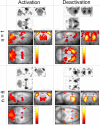
Figure 2. Maps of SWD-Related Changes in CBV
Left: activatio_n_ = increase of CBV; right: deactivatio_n_ = decrease of CBV. Top: typical example of activation/deactivation pattern obtained for a single animal (n = 1, p < 0.001, FWE corrected). Bottom: activation/deactivation pattern of the group of animals (n = 6, fixed effect analysis, p < 0.05, FWE corrected). Structures activated at the group level are listed in Table 1.
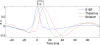
Figure 3. Hemodynamic Response Functions of (De)activated Structures during SWDs
(A) Hemodynamics of activated and deactivated structures during SWDs. HRFs for the different ROIs were generated using the median value (see Table 2) of parameters of a truncated hemodynamic model (see Equation 1) adjusted to the ROI time series. (B) From prior values (in blue) of hemodynamic parameters (see Table 2), the effect of each parameter to explain the behaviour of the HRF in S1BF (in green) was evaluated by changing the parameters to their value estimated in S1BF, one at a time. The abnormally slow hemodynamics in S1BF is primarily explained by the strong decrease in the autoregulation constant of the CBF on the vasodilatation (in cyan).
Figure 4. Functional Connectivity Estimated from Granger Causality
Oriented networks estimated using the linear measure of Granger causality for each animal (left) and for the group (right), without (top) and after (bottom) hemodynamic deconvolution. For each pair of regions (X, Y), the directionality and colour of the arrows indicate the sign and statistical significance (obtained from surrogates) of Fx→y − Fy→x (see Equation 4), respectively. See main text for details. St, striatum; Th, thalamus.
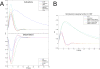
Figure 5. Dynamic Causal Modelling
(A) Example showing how DCM (model 1) fitted measured data from a session containing four seizures. (B) Model comparison using the negative free energy (for clarity, the average over the models of the negative energy has been removed). Top: at the group level, the models 1–5 assuming S1BF as being a driver are the most plausible (model 3 is the most plausible at the group level, mainly because of the high value of its evidence in rat 2). Bottom: this result at the group level was found in all rats when pooling over each class. However, in rats 3 and 5, a model assuming the striatum as a driver was found the most plausible (in rat 5, this finding was not significant, i.e., difference of negative energy with a model assuming S1BF as being a driver was lower than three). (C) Neuronal and hemodynamic kernels at the group level obtained from median value of model parameters estimated at the individual level for the most plausible model (model 3, see [B]). (D) Extrinsic connectivity, obtained after averaging matrices A and C over the animals, for the most plausible model (model 3, see [B]).
Figure 6. Spike and Wave Complex Averaged over Seizures and Rats
The spike observed in S1BF precedes by 5.5 ms and by 10 ms (time to peak) those measured in the thalamus and in the striatum, respectively.
Figure 7. Direction of Information Transfer Estimated in iEEG from the Measure of Generalised Synchronisation
A significant and stable, among the first seconds of SWDs, driving effect was found from S1BF towards thalamus and striatum. No consistent directionality was found for the connection between striatum and thalamus.
Figure 8. Dynamic Causal Model: Architecture and State Equations
Input u corresponds to detected epileptic events in the EEG. All parameters of the models are estimated from data y (CBV-weighted fMRI signals) using a Bayesian framework. Different configurations of the interregional connectivity A as shown in the competing models are used to estimate the putative neural driver, based on Bayesian model comparison. The 15 possible unidirectional models were generated from the five models shown in this figure using permutations on the ROI names (S1BF driver: models 1–5; thalamus driver: models 6–10; striatum driver: models 11–15). See main text for additional details.
Comment in
- Causal modelling and brain connectivity in functional magnetic resonance imaging.
Friston K. Friston K. PLoS Biol. 2009 Feb 17;7(2):e33. doi: 10.1371/journal.pbio.1000033. PLoS Biol. 2009. PMID: 19226186 Free PMC article.
Similar articles
- Estimation of the effective and functional human cortical connectivity with structural equation modeling and directed transfer function applied to high-resolution EEG.
Astolfi L, Cincotti F, Mattia D, Salinari S, Babiloni C, Basilisco A, Rossini PM, Ding L, Ni Y, He B, Marciani MG, Babiloni F. Astolfi L, et al. Magn Reson Imaging. 2004 Dec;22(10):1457-70. doi: 10.1016/j.mri.2004.10.006. Magn Reson Imaging. 2004. PMID: 15707795 - Where fMRI and electrophysiology agree to disagree: corticothalamic and striatal activity patterns in the WAG/Rij rat.
Mishra AM, Ellens DJ, Schridde U, Motelow JE, Purcaro MJ, DeSalvo MN, Enev M, Sanganahalli BG, Hyder F, Blumenfeld H. Mishra AM, et al. J Neurosci. 2011 Oct 19;31(42):15053-64. doi: 10.1523/JNEUROSCI.0101-11.2011. J Neurosci. 2011. PMID: 22016539 Free PMC article. - Dynamic fMRI and EEG recordings during spike-wave seizures and generalized tonic-clonic seizures in WAG/Rij rats.
Nersesyan H, Hyder F, Rothman DL, Blumenfeld H. Nersesyan H, et al. J Cereb Blood Flow Metab. 2004 Jun;24(6):589-99. doi: 10.1097/01.WCB.0000117688.98763.23. J Cereb Blood Flow Metab. 2004. PMID: 15181366 - Human brain mapping: hemodynamic response and electrophysiology.
Shibasaki H. Shibasaki H. Clin Neurophysiol. 2008 Apr;119(4):731-43. doi: 10.1016/j.clinph.2007.10.026. Epub 2008 Jan 9. Clin Neurophysiol. 2008. PMID: 18187361 Review. - Pathophysiology of absence epilepsy: Insights from genetic models.
Depaulis A, Charpier S. Depaulis A, et al. Neurosci Lett. 2018 Feb 22;667:53-65. doi: 10.1016/j.neulet.2017.02.035. Epub 2017 Feb 16. Neurosci Lett. 2018. PMID: 28216336 Review.
Cited by
- Intracranial EEG-Based Directed Functional Connectivity in Alpha to Gamma Frequency Range Reflects Local Circuits of the Human Mesiotemporal Network.
Novitskaya Y, Schulze-Bonhage A, David O, Dümpelmann M. Novitskaya Y, et al. Brain Topogr. 2024 Oct 22;38(1):10. doi: 10.1007/s10548-024-01084-w. Brain Topogr. 2024. PMID: 39436471 Free PMC article. - A Randomization-Based, Model-Free Approach to Functional Neuroimaging: A Proof of Concept.
Mazor M, Mukamel R. Mazor M, et al. Entropy (Basel). 2024 Sep 2;26(9):751. doi: 10.3390/e26090751. Entropy (Basel). 2024. PMID: 39330084 Free PMC article. - A Comparative Study of Causality Detection Methods in Root Cause Diagnosis: From Industrial Processes to Brain Networks.
Zhou S, Cai H, Chen H, Ye L. Zhou S, et al. Sensors (Basel). 2024 Jul 29;24(15):4908. doi: 10.3390/s24154908. Sensors (Basel). 2024. PMID: 39123955 Free PMC article. - Visual system structural and functional connections during face viewing in body dysmorphic disorder.
Wong WW, Peel H, Cabeen R, Diaz-Fong JP, Feusner JD. Wong WW, et al. bioRxiv [Preprint]. 2024 Jul 16:2024.07.12.603273. doi: 10.1101/2024.07.12.603273. bioRxiv. 2024. PMID: 39071433 Free PMC article. Preprint. - Causalized Convergent Cross Mapping and Its Implementation in Causality Analysis.
Sun B, Deng J, Scheel N, Zhu DC, Ren J, Zhang R, Li T. Sun B, et al. Entropy (Basel). 2024 Jun 24;26(7):539. doi: 10.3390/e26070539. Entropy (Basel). 2024. PMID: 39056902 Free PMC article.
References
- Dayan P, Abbott LF. Theoretical neuroscience: computational and mathematical modeling of neural systems. Cambridge (Massachusetts): Massachusetts Institute of Technology Press; 2001. 460
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–716. - PubMed
- McIntosh AR. Towards a network theory of cognition. Neural Netw. 2000;13:861–870. - PubMed
- Friston K. Learning and inference in the brain. Neural Netw. 2003;16:1325–1352. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials