Inhibitory effects of progesterone differ in dendritic cells from female and male rodents - PubMed (original) (raw)
Inhibitory effects of progesterone differ in dendritic cells from female and male rodents
Cherié L Butts et al. Gend Med. 2008 Dec.
Abstract
Background: Steroid hormones, such as progesterone, are known to have immunomodulatory effects. Our research group previously reported direct effects of progesterone on dendritic cells (DCs) from female rodents. Primarily affecting mature DC function, progesterone effects included inhibition of proinflammatory cytokine secretion, downregulation of cell surface marker (major histocompatibility complex class II, CD80) expression, and decreased T-cell proliferative capacity, and were likely mediated through progesterone receptor (PR) because the PR antagonist RU486 reversed these effects.
Objective: The goal of this study was to assess differences in response to progesterone by DCs from female and male rodents.
Methods: Using real-time reverse-transcriptase polymerase chain reaction, transcriptional expression of steroid hormone receptors was measured in immature bone marrow-derived DCs (BMDCs) from male and female rats. Expression of steroid hormone receptor protein was also assessed in these cells using flow cytometry and fluorescence microscopy. To evaluate functional differences between BMDCs from female and male rats in response to the steroid hormone progesterone, levels of secreted cytokines were measured using enzyme-linked immunosorbent assay.
Results: Higher numbers of immature BMDCs from males expressed glucocorticoid receptor (GR) and androgen receptor (AR) proteins compared with females (males vs females, mean [SD]: GR = 68.75 [7.27] vs 43.61 [13.97], P = NS; AR = 75.99 [15.38] vs 8.25 [1.88], P = 0.002), whereas higher numbers of immature BMDCs from females expressed PR protein compared with males (females vs males: PR = 74.19 [12.11] vs 14.14 [4.55], P = 0.043). These differences were not found at the level of transcription (females vs males: GR = 0.088 vs 0.073, P = NS; AR = 0.076 vs 0.069, P = NS; PR = 0.075 vs 0.065, P = NS). Compared with those from females, mature BMDCs from males produced higher quantities of cytokines (tumor necrosis factor-alpha [TNF-alpha], interleukin [IL]-1beta, IL-10) (females vs males: TNF-alpha = 920.0 [79.25] vs 1100.61 [107.97], P = NS; IL-1beta = 146.60 [38.04] vs 191.10 [10.47], P = NS; IL-10 = 167.25 [4.50] vs 206.15 [23.48], P = NS). Conversely, BMDCs from females were more sensitive to progesterone, as indicated by a more dramatic reduction in proinflammatory cytokine secretion (females vs males, highest concentration of progesterone: TNF-alpha = 268.94 [28.59] vs 589.91 [100.98], P = 0.04; IL-1beta = 119.50 [10.32] vs 154.35 [6.22], P = NS).
Conclusions: These findings suggest that progesterone effects on DCs in rodents may be more pronounced in females than in males, and this is likely due to differences in PR protein expression. Our observations may help elucidate disparities in the incidence and severity of autoimmune disorders between females and males, and the role specific steroid hormones play in regulating immune responses.
Figures
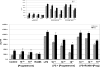
Figure 1
Transcriptional expression of steroid hormone receptor by immune cells from female and male rats (n = 9 each). Total RNA from cultured cells was isolated and analyzed for expression of the steroid hormone receptor genes for glucocorticoid (GR), androgen (AR), and progesterone (PR). Total RNA from CD11 c+ dendritic cells (DCs) and T lymphocytes was also isolated from splenic tissue for comparison to bone marrow–derived DCs (BMDCs). Values are presented as the mean (SO) of the relative fold induction of the specified gene.
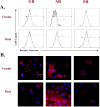
Figure 2
Expression of steroid hormone receptor protein by immature bone marrow–derived dendritic cells (BMDCs) from female and male rats (n = 7 each). (A) Representative histograms show proportions of immature BMDCs expressing receptors for glucocorticoid (GR), androgen (AR), and progesterone (PR). (B) Representative fluorescent micrographs show immature BMDC expression of steroid hormone receptor (red) and were counterstained (blue) with DAPI (4′-6-diamidino-2-phenylindole) to identify nuclei of cells.
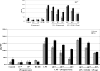
Figure 3
Production of proinflammatory cytokine tumor necrosis factor-α by lipopolysaccharide (LPS)-stimulated bone marrow–derived dendritic cells from female and male rats (n = 8 each). Cells were stimulated to maturity with LPS and treated with progesterone or a combination of progesterone and the progesterone receptor antagonist RU486. Values are presented as mean (SD). *p ≤ 0.05 versus LPS alone.
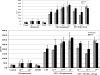
Figure 4
Production of proinflammatory cytokine interleukin-1β by lipopolysaccharide (LPS)-stimulated bone marrow–derived dendritic cells from female and male rats (n = 8 each). Cells were stimulated to maturity with LPS and treated with progesterone or a combination of progesterone and the progesterone receptor antagonist RU486. Values are presented as mean (SD). *p ≤ 0.05 versus LPS alone.
Figure 5
Production of T-helper 2 response-promoting cytokine interleukin-10 by lipopolysaccharide (LPS)-stimulated bone marrow–derived dendritic cells from female and male rats (n = 6 each). Cells were stimulated to maturity with LPS and treated with progesterone or a combination of progesterone and the progesterone receptor antagonist RU486. Values are presented as mean (SD).
Similar articles
- Progesterone as an Anti-Inflammatory Drug and Immunomodulator: New Aspects in Hormonal Regulation of the Inflammation.
Fedotcheva TA, Fedotcheva NI, Shimanovsky NL. Fedotcheva TA, et al. Biomolecules. 2022 Sep 14;12(9):1299. doi: 10.3390/biom12091299. Biomolecules. 2022. PMID: 36139138 Free PMC article. Review. - Progesterone inhibits mature rat dendritic cells in a receptor-mediated fashion.
Butts CL, Shukair SA, Duncan KM, Bowers E, Horn C, Belyavskaya E, Tonelli L, Sternberg EM. Butts CL, et al. Int Immunol. 2007 Mar;19(3):287-96. doi: 10.1093/intimm/dxl145. Epub 2007 Feb 7. Int Immunol. 2007. PMID: 17289656 - The effect of high mobility group box-1 protein on splenic dendritic cell maturation in rats.
Zhu XM, Yao YM, Liang HP, Xu S, Dong N, Yu Y, Sheng ZY. Zhu XM, et al. J Interferon Cytokine Res. 2009 Oct;29(10):677-86. doi: 10.1089/jir.2008.0104. J Interferon Cytokine Res. 2009. PMID: 19642897 - Effects of histamine and its antagonists on murine T-cells and bone marrow-derived dendritic cells.
Hu X, Zafar MI, Gao F. Hu X, et al. Drug Des Devel Ther. 2015 Aug 21;9:4847-60. doi: 10.2147/DDDT.S89792. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26346531 Free PMC article. - Steroids in Stroke with Special Reference to Progesterone.
Guennoun R, Zhu X, Fréchou M, Gaignard P, Slama A, Liere P, Schumacher M. Guennoun R, et al. Cell Mol Neurobiol. 2019 May;39(4):551-568. doi: 10.1007/s10571-018-0627-0. Epub 2018 Oct 9. Cell Mol Neurobiol. 2019. PMID: 30302630 Review.
Cited by
- Diagnostic tests for progestogen hypersensitivity.
Alonso Bello CD, González Guzmán OP, Moncayo Coello CV, Rojo Gutiérrez MI, Castrejón Vázquez MI. Alonso Bello CD, et al. Front Allergy. 2024 Apr 24;5:1384140. doi: 10.3389/falgy.2024.1384140. eCollection 2024. Front Allergy. 2024. PMID: 38720769 Free PMC article. Review. - Liposome-encapsulated progesterone efficiently suppresses B-lineage cell proliferation.
Seki T, Suzuki R, Ohshima S, Manabe Y, Onoue S, Hoshino Y, Yasuda A, Ito R, Kawada H, Ishimoto H, Shiina T, Kametani Y. Seki T, et al. Biochem Biophys Rep. 2024 Apr 11;38:101710. doi: 10.1016/j.bbrep.2024.101710. eCollection 2024 Jul. Biochem Biophys Rep. 2024. PMID: 38638674 Free PMC article. - CFP1 governs uterine epigenetic landscapes to intervene in progesterone responses for uterine physiology and suppression of endometriosis.
Yang SC, Park M, Hong KH, La H, Park C, Wang P, Li G, Chen Q, Choi Y, DeMayo FJ, Lydon JP, Skalnik DG, Lim HJ, Hong SH, Park SH, Kim YS, Kim HR, Song H. Yang SC, et al. Nat Commun. 2023 Jun 3;14(1):3220. doi: 10.1038/s41467-023-39008-0. Nat Commun. 2023. PMID: 37270588 Free PMC article. - Progesterone as an Anti-Inflammatory Drug and Immunomodulator: New Aspects in Hormonal Regulation of the Inflammation.
Fedotcheva TA, Fedotcheva NI, Shimanovsky NL. Fedotcheva TA, et al. Biomolecules. 2022 Sep 14;12(9):1299. doi: 10.3390/biom12091299. Biomolecules. 2022. PMID: 36139138 Free PMC article. Review. - Sex and Gender Differences in Bacterial Infections.
Dias SP, Brouwer MC, van de Beek D. Dias SP, et al. Infect Immun. 2022 Oct 20;90(10):e0028322. doi: 10.1128/iai.00283-22. Epub 2022 Sep 19. Infect Immun. 2022. PMID: 36121220 Free PMC article. Review.
References
- Verthelyi D. Sex hormones as immunomodulators in health and disease. Int Immunopharmacol. 2001;1:983–993. - PubMed
- Butts C, Sternberg E. Different approaches to understanding autoimmune rheumatic diseases: The neuroimmunoendocrine system. Best Pract Res Clin Rheumatol. 2004;18:125–139. - PubMed
- Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–780. - PubMed
- Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1:199–205. - PubMed
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous