The bacterial fermentation product butyrate influences epithelial signaling via reactive oxygen species-mediated changes in cullin-1 neddylation - PubMed (original) (raw)
The bacterial fermentation product butyrate influences epithelial signaling via reactive oxygen species-mediated changes in cullin-1 neddylation
Amrita Kumar et al. J Immunol. 2009.
Abstract
The human enteric flora plays a significant role in intestinal health and disease. Populations of enteric bacteria can inhibit the NF-kappaB pathway by blockade of IkappaB-alpha ubiquitination, a process catalyzed by the E3-SCF(beta-TrCP) ubiquitin ligase. The activity of this ubiquitin ligase is regulated via covalent modification of the Cullin-1 subunit by the ubiquitin-like protein NEDD8. We previously reported that interaction of viable commensal bacteria with mammalian intestinal epithelial cells resulted in a rapid and reversible generation of reactive oxygen species (ROS) that modulated neddylation of Cullin-1 and resulted in suppressive effects on the NF-kappaB pathway. Herein, we demonstrate that butyrate and other short chain fatty acids supplemented to model human intestinal epithelia in vitro and human tissue ex vivo results in loss of neddylated Cul-1 and show that physiological concentrations of butyrate modulate the ubiquitination and degradation of a target of the E3- SCF(beta-TrCP) ubiquitin ligase, the NF-kappaB inhibitor IkappaB-alpha. Mechanistically, we show that physiological concentrations of butyrate induces reactive oxygen species that transiently alters the intracellular redox balance and results in inactivation of the NEDD8-conjugating enzyme Ubc12 in a manner similar to effects mediated by viable bacteria. Because the normal flora produces significant amounts of butyrate and other short chain fatty acids, these data provide a functional link between a natural product of the intestinal normal flora and important epithelial inflammatory and proliferative signaling pathways.
Conflict of interest statement
Disclosures
The authors have no financial conflict of interest.
Figures
FIGURE 1
Bacterial fermentation products at physiological pH and concentration cause loss of Cul-1 neddylation. A, Immunoblot of Cul-1 and NEDD8 from T84 model epithelia treated for up to 90 min with physiological concentrations of butyrate. B, Immunoblot of Cul-1 from HeLa cell lines (top panel) and HL-60 differentiated into macrophage-like cells treated for up to 60 min with physiological concentrations of butyrate. C, Immunoblot of Cul-1 from T84 model epithelia treated for up to 1 h with an effective dose of butyrate, washed, and incubated for 30 min and 1 h longer in HBSS+. D, Immunoblot of Cul-1 from T84 epithelial cells treated with HBSS+ supplemented with butyrate and other organic acids as indicated for 1 h. Decreasing acid concentration is marked (open wedge; 0–14 mM) and has an inverse relationship to pH (solid wedge; pH 4.0–7.5). E, Immunoblot of Cul-1 from T84 model epithelia treated with HBSS+ or HBSS+ supplemented with butyrate for 1 h at the indicated concentrations. The effective concentration of HBSS+ or HBSS+ supplemented with butyrate was buffered to the pH as indicated below each panel. F, Immunoblot of Cul-1 from 3-mm human distal colonic mucosal punches treated for 1 h in HBSS+ supplemented with butyrate.
FIGURE 2
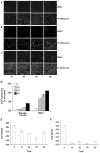
Loss of Cul-1 neddylation results in inhibition of TNF-_α_-induced p65 nuclear translocation and I_κ_B-_α_ubiquitination and degradation. The effective concentration of TNF-α and purified flagellin used for these experiments are 10 and 100 ng/ml, respectively. A, Butyrate prevents TNF-_κ_-induced p65 nuclear translocation. Confocal image of cytoplasmic vs nuclear immunofluorescent staining of p65 (Rockland) in HeLa cells treated for 1 h with HBSS+ supplemented with butyrate or lactate at the indicated concentrations and challenged with TNF-α for 20 min. B, Butyrate inhibits activity of a NF-_κ_B-luciferase reporter. Lysates from HeLa cells transfected with a NF-_κ_B-luciferase reporter plasmid and treated in the presence of butyrate with TNF-α for 5 h were assayed for luciferase activity. Data represent the mean of three independent assays and are shown as percent TNF-_α_-induced NF-_κ_B-Luc. C, Butyrate does not attenuate polyubiquitination of proteins. The level of protein ubiquitination in cellular extracts of Caco-2 cells pretreated with 2.5 mM butyrate for 1 h before addition of MG-262 for additional time was determined by immunoblotting with anti-ubiquitin conjugate Ab (Affinity Research). D, Immunoblots of whole cell extracts with anti-total-I_κ_B-α Ab from Caco-2 cells pretreated with the proteasomal inhibitor MG-262 (500 ng/ml) or HBSS+ for 40 min before a 1-h incubation with 5 mM butyrate and subsequent TNF-α (T; 10 ng/ml) or flagellin (F; 100 ng/ml) challenge for 5 or 15 min. Ubiquitinated I_κ_B-α species are marked. Equal loading was confirmed by _β_-tubulin immunoblots. E, Immunoblots of whole cell extracts with anti-phospho-I_κ_B-α Ab from Caco-2 cells pretreated with the proteasomal inhibitor MG-262 (500 ng/ml) for 40 min before a 1-h incubation with 5 mM butyrate and subsequent TNF-α (T; 10 ng/ml) or flagellin (F; 100 ng/ml) challenge for 5 or 15 min.
FIGURE 3
Butyrate induces generation of epithelial ROS and causes redox changes. IEC-6 cells were treated with HBSS with or without 10 mM butyrate, washed, and incubated with 5 _μ_M of DCF (A), 10 _μ_M DHE (B), and 10 _μ_M Mito-Sox Red (C). Fluorescence was captured by confocal laser-scanning microscopy (Zeiss). D, Kinetics of butyrate-induced ROS generation in epithelial cells. Confluent Caco-2 cells were preloaded with 100 _μ_M DCF and then ROS production was measured upon colonization with Lactobacillus (MOI = 1) or treatment with 10 mM butyrate. Data represent the mean of three independent assays and are shown as percent induction of ROS upon untreated controls. E and F, Butyrate cause oxidation of Trx1 and Trx2 pools. Confluent Caco2 cells were treated with PBS with or without butyrate as indicated over a time course. Graphs show the redox potential (Eh) for Trx1 and Trx2, respectively. Data are represented as means ± SE.
FIGURE 4
Butyrate-mediated oxidation of Ubc12. A, Butyrate inhibits formation of the NEDD8~Ubc12 thiolester bond of endogenous Ubc12. Western blot analysis with Ubc12-specific antisera of whole cell protein extracted from HeLa cells treated with butyrate for 1 h at the indicated dose and lysed in SDS lysis buffer without (− DTT) or with (+DTT) reducing agents. The asterisk marks the 30-kDa DTT-sensitive NEDD8~Ubc12 thiolester form and the arrow indicates the oxidized forms of Ubc12. B, NAC and DPI prevents butyrate-mediated loss of Cul-1 neddylation. HeLa cells pretreated with NAC or DPI for 60 min and subsequently treated with 5 mM butyrate for 1 h were analyzed by immunoblotting with anti-Cul-1 Ab.
Comment in
- Comment on "The bacterial fermentation product butyrate influences epithelial signaling via reactive oxygen species-mediated changes in cullin-1 neddylation".
Png E, Siak JK, Chew J, Tong L. Png E, et al. J Immunol. 2009 Oct 1;183(7):4146. doi: 10.4049/jimmunol.0990077. J Immunol. 2009. PMID: 19767563 No abstract available.
Similar articles
- Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species.
Kumar A, Wu H, Collier-Hyams LS, Hansen JM, Li T, Yamoah K, Pan ZQ, Jones DP, Neish AS. Kumar A, et al. EMBO J. 2007 Oct 31;26(21):4457-66. doi: 10.1038/sj.emboj.7601867. Epub 2007 Oct 4. EMBO J. 2007. PMID: 17914462 Free PMC article. - Cutting edge: bacterial modulation of epithelial signaling via changes in neddylation of cullin-1.
Collier-Hyams LS, Sloane V, Batten BC, Neish AS. Collier-Hyams LS, et al. J Immunol. 2005 Oct 1;175(7):4194-8. doi: 10.4049/jimmunol.175.7.4194. J Immunol. 2005. PMID: 16177058 - Comment on "The bacterial fermentation product butyrate influences epithelial signaling via reactive oxygen species-mediated changes in cullin-1 neddylation".
Png E, Siak JK, Chew J, Tong L. Png E, et al. J Immunol. 2009 Oct 1;183(7):4146. doi: 10.4049/jimmunol.0990077. J Immunol. 2009. PMID: 19767563 No abstract available. - Control of IkappaBalpha proteolysis by the ubiquitin-proteasome pathway.
Tanaka K, Kawakami T, Tateishi K, Yashiroda H, Chiba T. Tanaka K, et al. Biochimie. 2001 Mar-Apr;83(3-4):351-6. doi: 10.1016/s0300-9084(01)01237-8. Biochimie. 2001. PMID: 11295496 Review. - Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity.
Karin M, Ben-Neriah Y. Karin M, et al. Annu Rev Immunol. 2000;18:621-63. doi: 10.1146/annurev.immunol.18.1.621. Annu Rev Immunol. 2000. PMID: 10837071 Review.
Cited by
- Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: new insight into the probiotics for the gut-liver axis.
Endo H, Niioka M, Kobayashi N, Tanaka M, Watanabe T. Endo H, et al. PLoS One. 2013 May 16;8(5):e63388. doi: 10.1371/journal.pone.0063388. Print 2013. PLoS One. 2013. PMID: 23696823 Free PMC article. - Role of gut microbiota in intestinal wound healing and barrier function.
Alam A, Neish A. Alam A, et al. Tissue Barriers. 2018;6(3):1539595. doi: 10.1080/21688370.2018.1539595. Epub 2018 Nov 7. Tissue Barriers. 2018. PMID: 30404570 Free PMC article. Review. - Reactive oxygen production induced by the gut microbiota: pharmacotherapeutic implications.
Jones RM, Mercante JW, Neish AS. Jones RM, et al. Curr Med Chem. 2012;19(10):1519-29. doi: 10.2174/092986712799828283. Curr Med Chem. 2012. PMID: 22360484 Free PMC article. Review. - Butyrate administration is not sufficient to improve immune reconstitution in antiretroviral-treated SIV-infected macaques.
Ortiz AM, Simpson J, Langner CA, Baker PJ, Aguilar C, Brooks K, Flynn JK, Vinton CL, Rahmberg AR, Hickman HD, Brenchley JM. Ortiz AM, et al. Sci Rep. 2022 May 6;12(1):7491. doi: 10.1038/s41598-022-11122-x. Sci Rep. 2022. PMID: 35523797 Free PMC article. - Microbes in gastrointestinal health and disease.
Neish AS. Neish AS. Gastroenterology. 2009 Jan;136(1):65-80. doi: 10.1053/j.gastro.2008.10.080. Epub 2008 Nov 19. Gastroenterology. 2009. PMID: 19026645 Free PMC article. Review.
References
- Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. - PubMed
- Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. - PubMed
- Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. - PubMed
- Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–1064. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 AI064462/AI/NIAID NIH HHS/United States
- DK-71604/DK/NIDDK NIH HHS/United States
- R01 DK071604-04/DK/NIDDK NIH HHS/United States
- R01 DK071604/DK/NIDDK NIH HHS/United States
- AI-64462/AI/NIAID NIH HHS/United States
- R01 AI064462-04/AI/NIAID NIH HHS/United States
- R01 AI064462/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous