Molecular architecture of the centriole proteome: the conserved WD40 domain protein POC1 is required for centriole duplication and length control - PubMed (original) (raw)
Molecular architecture of the centriole proteome: the conserved WD40 domain protein POC1 is required for centriole duplication and length control
Lani C Keller et al. Mol Biol Cell. 2009 Feb.
Abstract
Centrioles are intriguing cylindrical organelles composed of triplet microtubules. Proteomic data suggest that a large number of proteins besides tubulin are necessary for the formation and maintenance of a centriole's complex structure. Expansion of the preexisting centriole proteome from the green alga Chlamydomonas reinhardtii revealed additional human disease genes, emphasizing the significance of centrioles in normal human tissue homeostasis. We found that two classes of ciliary disease genes were highly represented among the basal body proteome: cystic kidney disease (especially nephronophthisis) syndromes, including Meckel/Joubert-like and oral-facial-digital syndrome, caused by mutations in CEP290, MKS1, OFD1, and AHI1/Jouberin proteins and cone-rod dystrophy syndrome genes, including UNC-119/HRG4, NPHP4, and RPGR1. We further characterized proteome of the centriole (POC) 1, a highly abundant WD40 domain-containing centriole protein. We found that POC1 is recruited to nascent procentrioles and localizes in a highly asymmetrical pattern in mature centrioles corresponding to sites of basal-body fiber attachment. Knockdown of POC1 in human cells caused a reduction in centriole duplication, whereas overexpression caused the appearance of elongated centriole-like structures. Together, these data suggest that POC1 is involved in early steps of centriole duplication as well as in the later steps of centriole length control.
Figures
Figure 1.
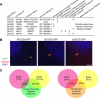
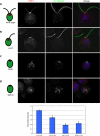
Expansion of the Chlamydomonas centriole proteome reveals additional human disease genes. (A) Table of newly discovered POCs and BUGs. Version 3 gene identification numbers are as specified in version 3.0 of the Chlamydomonas genome sequence, available at the Joint Genomes Institute website at
http://genome.jgi-psf.org/Chlre3/Chlre3.home.html
. Table also indicates protein name, human Refseq ID numbers, localization of proteins to other proteomes of interest, GFP localization to centrioles in human cells, and any associated human diseases. (B) Localization of GFP-fusion proteins corresponding to human homologues of Chlamydomonas centriole candidate proteins (POC20/FAP124, BUG30/Ro/SSA, and BUG21/Mns1) after transient transfection into HeLa cells. γ-Tubulin antibody stain shows centrosomes; DAPI stain shows DNA. Each GFP-fusion protein colocalizes to a pair of dots within the centrosome in HeLa cells, confirming centriolar localization. Bar, 5 μm. (C) Venn diagrams illustrating the overlap of POC and BUG proteins with the Chlamydomonas flagellar proteome (Pazour et al., 2005) and with the Tetrahymena centriole proteome (Kilburn et al., 2007). Percentage of POC and BUG proteins overlapping with the specified proteomes are indicated.
Figure 2.
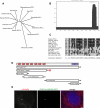
POC1 is a conserved centriole protein that is recruited to the centriole through the N-terminal WD40 repeat domain. (A) Phylogenetic unrooted tree showing that POC1 is conserved from Chlamydomonas to humans. The POC1 protein underwent a gene duplication in vertebrates as shown. (B) Coiled-coil prediction analysis of POC1 illustrates that there is a coiled-coil domain at the C terminus of the protein (COILS server; ch.EMBnet.org). (C) The POC1 domain is a conserved sequence at the C terminus of all conserved POC1 sequences. The POC1 consensus sequence is indicated at the bottom of the sequence alignment. (D) POC1 domain architecture. The WD40 repeat domain contains seven repeats (labeled wd1–wd7) and is sufficient for localization to centrioles in human cells (depicted by red star). Numbers represent amino acid positions. Coiled-coil domain indicated by purple box. (E) POC1-WD40-GFP (green; middle) colocalizes with γ-tubulin (red; left) in transiently transfected HeLa cells. Merged image with nuclear stain (right; blue, DAPI). Bar, 5 μm.
Figure 3.
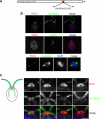
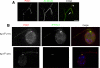
Chlamydomonas POC1 immunofluorescence. (A) Illustration depicting the position and sequence of the POC1 peptide that was used to create the Chlamydomonas POC1 antibody. Numbers represent amino acid positions. (B) POC1 (red) localizes to the basal bodies of wild-type Chlamydomonas cells, as shown by colocalization with acetylated tubulin antibody (top; green). Merged image is with nuclear stain (blue, DAPI). The middle panel indicates that POC1 (red) colocalizes with centrin (green) antibody only at the basal body. POC1 is absent from the centrin-based nuclear-attachment fibers. The bottom panel demonstrates that POC1 (red) costains with acetylated tubulin (green) to mark two centrioles at each pole of the mitotic spindle. Merged image is stained with a nuclear stain (blue, DAPI). (C) POC1 localizes to centrioles in a distinct pattern. Illustration of Chlamydomonas basal bodies (red), flagella (green), and inner rootlet microtubules (smaller green lines). The boxed region represents the area of high magnification in the next panels. Merged images demonstrate that POC1 (red) forms a cylindrical structure at the base of the flagella (green). Bar, 1 μm.
Figure 4.
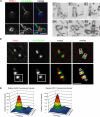
POC1 localizes to newly duplicating centrioles in both Chlamydomonas and in human cells. (A) Chlamydomonas POC1 (red) localizes to both mature basal bodies and proBBs, indicated by the appearance of four dots at the base of the flagella (green) as shown in the higher magnification images. Bar, 1 μm. (B) Immuno-EM of Chlamydomonas basal body sections. Cells were labeled with anti-POC1 antibody and gold-conjugated secondary antibodies. Localization of POC1 to probasal bodies is highlighted with arrowheads. B1–3 are serial sections as are B4 and B5. Bar, 250 nm. (C) Immunofluorescence of stably expressing human POC1-GFP (green). HeLa cells with centrosomes stained with Cetn2 (red). Merged images show two mature centrioles (top) and two mature centrioles along with their newly duplicating daughter centrioles from their proximal ends (bottom). Overlay images demonstrate proximal and distal ends of the mature centrioles along with newly duplicating daughter centrioles. Bar, 1 μm. (D) Fluorescence intensity quantification of boxed images in C. Large peak represents mature basal body; smaller peak represents newly duplicating daughter centriole. Note the smaller peak in the Cetn2 graph is shifted farther (more distal) from the mature centriole than the smaller peak in the POC1 graph.
Figure 5.
POC1 localization in Chlamydomonas basal body mutants. (A) Wild-type Chlamydomonas cells stained with POC1 (red) and acetylated tubulin (green). Note staining at the base of the flagella (green). (B) uni3 cells stained with POC1 (red) and acetylated-tubulin (green), demonstrating that POC1 stains doublet microtubules. (C) bld2 cells stained with POC1 (red) and acetylated-tubulin (green), indicating that POC1 stains singlet microtubules. (D) bld10 cells stained with POC1 (red) and acetylated tubulin (green) showing that POC1 always colocalizes with acetylated tubulin positive centrioles. Merged images were stained with a nuclear stain (blue, DAPI). (E) Histogram of POC1 staining intensity indicating reduced POC1 recruitment levels in mutant basal bodies.
Figure 6.
Immuno-EM of Chlamydomonas POC1 reveals localization to triplet microtubules and sites of fiber attachment. (A1–A4) Immuno-EM of basal body serial sections showing POC1 localization throughout the length of the triplet microtubules. (B1–B3) Serial sections showing POC1 localization to triplet microtubules and rootlet microtubules (root mt's). (C) POC1 localizes to doublet microtubules of axonemes but is absent from central pair microtubules. (D and E) POC1 localizes to rootlet microtubules that are nearby and/or attached to centrioles. (F–H) Sections demonstrating that POC1 localizes to proBBs and occasionally at the cartwheel (cartwheel indicated by black box). (I1–I5) Serial sections through a longitudinally sectioned basal body. POC1 localizes to sites of rootlet microtubule attachment and sites where proximal and distal connecting fibers attach to the basal body (root mt's, rootlet microtubules; pcf proximal connecting fiber, arrow shows a high density of POC1 at rootlet microtubule connection and at proximal connecting fiber). All sections were labeled with anti-POC1 antibody and gold-conjugated secondary antibodies. Bar, 250 nm.
Figure 7.
Chlamydomonas POC1 associates with doublet microtubules in the axoneme and is not a component of the IFT machinery. (A) Flagellar splay assay demonstrates that POC1 (red) localizes specifically to the doublet microtubules (arrowhead) and is absent from central pair microtubules (arrow). Note basal body staining at the base of the splayed flagella with both POC1 and acetylated tubulin antibodies. Bar, 5 μm. (B) POC1 (red) localizes to flagella in fla10 ts Chlamydomonas cells at both 21 and 34°C, unlike the IFT protein IFT172.1, which is absent at 34°C.
Figure 8.
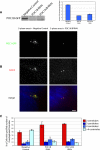
POC1 depletion leads to a reduction in centriole overduplication. (A) Western blot of POC1-GFP in RNAi-treated U2OS cell lysate. Ponceau stain was used as a loading control (data not shown). Quantification of Western blot normalized to 100%, indicated ∼75 and 60% knockdown of POC1B-GFP by POC1B siRNA and POC1A/B siRNA, respectively. (B) S phase-arrested U2OS cells have overduplicated centrioles, but when POC1 is depleted this, overduplication is suppressed (green, POC1B; red, Cetn2). (C) The percent of cells with overduplicated centrioles is reduced in the presence of POC1 siRNA, whereas the number of cells with wild-type number of two centrioles increases. Bar, 5 μm.
Figure 9.
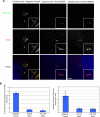
POC1 overexpression in human cells leads to elongated centriole-like structures, which is abolished when POC1 is knocked down by POC1 siRNA. (A) POC1B–GFP-expressing U2OS cells grown and treated with 3.2 μg/ml aphidicolin show a large percentage of cells with elongated centriole-like structures, which are POC1B-GFP positive and stain with Cetn2 (red). This overexpression phenotype is abolished in the presence of POC1 siRNA. (B) The percentage of cells with elongated centriole-like structures is dramatically reduced in the presence POC1 siRNA (left). The length of the elongated centriole-like structures was quantified and is also dramatically reduced in the presence of POC1 siRNA (right).
Similar articles
- Proteomic analysis of isolated chlamydomonas centrioles reveals orthologs of ciliary-disease genes.
Keller LC, Romijn EP, Zamora I, Yates JR 3rd, Marshall WF. Keller LC, et al. Curr Biol. 2005 Jun 21;15(12):1090-8. doi: 10.1016/j.cub.2005.05.024. Curr Biol. 2005. PMID: 15964273 - SAS-6 engineering reveals interdependence between cartwheel and microtubules in determining centriole architecture.
Hilbert M, Noga A, Frey D, Hamel V, Guichard P, Kraatz SH, Pfreundschuh M, Hosner S, Flückiger I, Jaussi R, Wieser MM, Thieltges KM, Deupi X, Müller DJ, Kammerer RA, Gönczy P, Hirono M, Steinmetz MO. Hilbert M, et al. Nat Cell Biol. 2016 Apr;18(4):393-403. doi: 10.1038/ncb3329. Epub 2016 Mar 21. Nat Cell Biol. 2016. PMID: 26999736 - The UNI1 and UNI2 genes function in the transition of triplet to doublet microtubules between the centriole and cilium in Chlamydomonas.
Piasecki BP, Silflow CD. Piasecki BP, et al. Mol Biol Cell. 2009 Jan;20(1):368-78. doi: 10.1091/mbc.e08-09-0900. Epub 2008 Nov 12. Mol Biol Cell. 2009. PMID: 19005206 Free PMC article. - Elucidation of basal body and centriole functions in Chlamydomonas reinhardtii.
Dutcher SK. Dutcher SK. Traffic. 2003 Jul;4(7):443-51. doi: 10.1034/j.1600-0854.2003.00104.x. Traffic. 2003. PMID: 12795689 Review. - Building the centriole.
Azimzadeh J, Marshall WF. Azimzadeh J, et al. Curr Biol. 2010 Sep 28;20(18):R816-25. doi: 10.1016/j.cub.2010.08.010. Curr Biol. 2010. PMID: 20869612 Free PMC article. Review.
Cited by
- Towards understanding centriole elimination.
Kalbfuss N, Gönczy P. Kalbfuss N, et al. Open Biol. 2023 Nov;13(11):230222. doi: 10.1098/rsob.230222. Epub 2023 Nov 15. Open Biol. 2023. PMID: 37963546 Free PMC article. Review. - Dzip1 and Fam92 form a ciliary transition zone complex with cell type specific roles in Drosophila.
Lapart JA, Gottardo M, Cortier E, Duteyrat JL, Augière C, Mangé A, Jerber J, Solassol J, Gopalakrishnan J, Thomas J, Durand B. Lapart JA, et al. Elife. 2019 Dec 10;8:e49307. doi: 10.7554/eLife.49307. Elife. 2019. PMID: 31821146 Free PMC article. - It takes two (centrioles) to tango.
Avidor-Reiss T, Fishman EL. Avidor-Reiss T, et al. Reproduction. 2019 Feb;157(2):R33-R51. doi: 10.1530/REP-18-0350. Reproduction. 2019. PMID: 30496124 Free PMC article. Review. - SAS-6 oligomerization: the key to the centriole?
Cottee MA, Raff JW, Lea SM, Roque H. Cottee MA, et al. Nat Chem Biol. 2011 Sep 19;7(10):650-3. doi: 10.1038/nchembio.660. Nat Chem Biol. 2011. PMID: 21931303 No abstract available. - An interaction network of inner centriole proteins organised by POC1A-POC1B heterodimer crosslinks ensures centriolar integrity.
Sala C, Würtz M, Atorino ES, Neuner A, Partscht P, Hoffmann T, Eustermann S, Schiebel E. Sala C, et al. Nat Commun. 2024 Nov 14;15(1):9857. doi: 10.1038/s41467-024-54247-5. Nat Commun. 2024. PMID: 39543170 Free PMC article.
References
- Adams M., Smith U. M., Logan C. V., Johnson C. A. Recent advances in the molecular pathology, cell biology and genetics of ciliopathies. J. Med. Genet. 2008;45:257–267. - PubMed
- Andersen J. S., Wilkinson C. J., Mayor T., Mortensen P., Nigg E. A., Mann M. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 2003;426:570–574. - PubMed
- Ansley S. J., et al. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425:628–633. - PubMed
- Azimzadeh J., Bornens M. Structure and duplication of the centrosome. J. Cell Sci. 2007;120:2139–2142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases