Rapid microglial response around amyloid pathology after systemic anti-Abeta antibody administration in PDAPP mice - PubMed (original) (raw)
Rapid microglial response around amyloid pathology after systemic anti-Abeta antibody administration in PDAPP mice
Jessica Koenigsknecht-Talboo et al. J Neurosci. 2008.
Abstract
Aggregation of amyloid-beta (Abeta) peptide in the brain in the form of neuritic plaques and cerebral amyloid angiopathy (CAA) is a key feature of Alzheimer's disease (AD). Microglial cells surround aggregated Abeta and are believed to play a role in AD pathogenesis. A therapy for AD that has entered clinical trials is the administration of anti-Abeta antibodies. One mechanism by which certain anti-Abeta antibodies have been proposed to exert their effects is via antibody-mediated microglial activation. Whether, when, or to what extent microglial activation occurs after systemic administration of anti-Abeta antibodies has not been fully assessed. We administered an anti-Abeta antibody (m3D6) that binds aggregated Abeta to PDAPP mice, an AD mouse model that was bred to contain fluorescent microglia. Three days after systemic administration of m3D6, there was a marked increase in both the number of microglial cells and processes per cell visualized in vivo by multiphoton microscopy. These changes required the Fc domain of m3D6 and were not observed with an antibody specific to soluble Abeta. These findings demonstrate that some effects of antibodies that recognize aggregated Abeta are rapid, involve microglia, and provide insight into the mechanism of action of a specific passive immunotherapy for AD.
Figures
Figure 1.
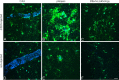
Microglia have altered morphology and cluster around amyloid plaques in PDAPP+/−;CX3CR1/GFP+/− mice. Three-dimensional reconstructed z-series stack images taken of cortical microglia in (A) PDAPP+/−;CX3CR1/GFP+/− mice at 3.5 months of age (in the absence of plaques), in (B) 14-month-old PDAPP+/−;CX3CR1/GFP+/− mice around amyloid plaques (blue), in (C) 14-month-old PDAPP+/−;CX3CR1/GFP+/− mice in areas lacking plaques, and in (D) 5-month-old PDAPP−/−;CX3CR1/GFP+/− mice. GFP-labeled microglia are green. Vessels are labeled with Texas Red dextran. Amyloid fluoresces blue after injection of methoxy-XO4. Scale bar, 20 μm.
Figure 2.
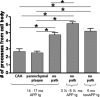
Decrease in the number of microglial processes around amyloid deposition. The number of processes extending from the cell body were counted in areas containing CAA, parenchymal amyloid plaques, and no amyloid pathology (no path). Microglia were compared in 14- to 17-month-old mice around CAA, parenchymal amyloid plaques, and in areas with no amyloid pathology as well as in 3.5- to 6.5-month-old PDAPP+/−;CX3CR1/GFP+/− mice lacking amyloid pathology and 5-month-old PDAPP−/−;CX3CR1/GFP+/− mice. Five mice were studied per age group in APP transgenic mice, and six fields of view were imaged in each animal. Six fields of view were studied in two non-APP transgenic mice. Data are presented as the mean ± SEM; *p < 0.001.
Figure 3.
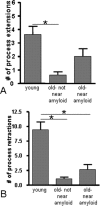
Decrease in microglial process movement in older PDAPP mice. The number of microglial process (A) extensions and (B) retractions were counted in young (3.5- to 6.5-month-old) and old (14- to 17-month-old) PDAPP+/−;CX3CR1/GFP+/− mice near and distant from amyloid pathology. Five mice were studied per age group, and six fields of view were imaged in each animal. Data are presented as the mean ± SEM; *p < 0.001.
Figure 4.
Peripheral m3D6 administration results in marked morphological changes in microglia. Three-dimensional reconstructed z-series stack images taken of 22-month-old PDAPP+/−;CX3CR1/GFP+/− mice injected with 500 μg of m3D6 (A–C), an anti-Aβ antibody, or not injected (D–F). GFP-labeled microglia are green. Fibrillar amyloid was labeled with methoxy-XO4 (blue). Scale bar, 20 μm.
Figure 5.
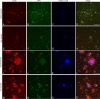
Iba-1 staining colocalizes with GFP-labeled microglia, and there is increased staining around plaques after m3D6 administration. Brain sections from 18-month-old PDAPP+/−;CX3CR1/GFP+/− mice were stained with Iba-1, and images were taken at low power (A–H) and high power (I–P). Iba-1-positive cells are in red (A, E, I, M), GFP labels CX3CR1-positive microglia (B, F, J, N), and methoxy-XO4 labels aggregated amyloid in blue (C, G, K, O). Merged images are also shown (D, G, L, P). Scale bars are 100 and 50 μm, respectively, for low-power and high-power images.
Figure 6.
CD45 colocalizes with GFP-labeled microglia around amyloid pathology, and there is increased staining around plaques after m3D6 administration. Brain sections from 18-month-old PDAPP+/−;CX3CR1/GFP+/− mice were stained with CD45, and images were taken at low power (A–H) and high power (I–P). CD45-positive cells are in red (A, E, I, M), GFP labels CX3CR1 positive microglia (B, F, J, N), and methoxy-XO4 labels aggregated amyloid in blue (C, G, K, O). Merged images are also shown (D, G, L, P). Scale bars are 100 and 50 μm, respectively, for low-power and high-power images.
Figure 7.
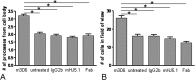
Microglial activation after antibody treatment requires recognition of aggregated Aβ and the Fc domain. The number of microglial processes (A) and the number of microglial cells (B) were counted in three-dimensional reconstructed z-series stack images in 18-month-old PDAPP+/−;CX3CR1/GFP+/− mice not injected (untreated) or injected with 500 μg of m3D6, 500 μg of IgG2b, 500 μg of mHJ5.1, and 500 μg of m3D6 Fab fragments. Four mice were studied per treatment group, and 6–10 fields of view were imaged in each animal. Data are presented as the mean ± SEM; *p < 0.001.
Similar articles
- An effector-reduced anti-β-amyloid (Aβ) antibody with unique aβ binding properties promotes neuroprotection and glial engulfment of Aβ.
Adolfsson O, Pihlgren M, Toni N, Varisco Y, Buccarello AL, Antoniello K, Lohmann S, Piorkowska K, Gafner V, Atwal JK, Maloney J, Chen M, Gogineni A, Weimer RM, Mortensen DL, Friesenhahn M, Ho C, Paul R, Pfeifer A, Muhs A, Watts RJ. Adolfsson O, et al. J Neurosci. 2012 Jul 11;32(28):9677-89. doi: 10.1523/JNEUROSCI.4742-11.2012. J Neurosci. 2012. PMID: 22787053 Free PMC article. Clinical Trial. - Microglia contributes to plaque growth by cell death due to uptake of amyloid β in the brain of Alzheimer's disease mouse model.
Baik SH, Kang S, Son SM, Mook-Jung I. Baik SH, et al. Glia. 2016 Dec;64(12):2274-2290. doi: 10.1002/glia.23074. Epub 2016 Sep 23. Glia. 2016. PMID: 27658617 - Human apolipoprotein E4 alters the amyloid-beta 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model.
Fryer JD, Simmons K, Parsadanian M, Bales KR, Paul SM, Sullivan PM, Holtzman DM. Fryer JD, et al. J Neurosci. 2005 Mar 16;25(11):2803-10. doi: 10.1523/JNEUROSCI.5170-04.2005. J Neurosci. 2005. PMID: 15772340 Free PMC article. - Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?
Merino JJ, Muñetón-Gómez V, Alvárez MI, Toledano-Díaz A. Merino JJ, et al. Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review. - Multiphoton in vivo imaging of amyloid in animal models of Alzheimer's disease.
Dong J, Revilla-Sanchez R, Moss S, Haydon PG. Dong J, et al. Neuropharmacology. 2010 Sep-Oct;59(4-5):268-75. doi: 10.1016/j.neuropharm.2010.04.007. Epub 2010 Apr 14. Neuropharmacology. 2010. PMID: 20398680 Free PMC article. Review.
Cited by
- Interaction between amyloid-β pathology and cortical functional columnar organization.
Beker S, Kellner V, Kerti L, Stern EA. Beker S, et al. J Neurosci. 2012 Aug 15;32(33):11241-9. doi: 10.1523/JNEUROSCI.2426-12.2012. J Neurosci. 2012. PMID: 22895708 Free PMC article. - An effector-reduced anti-β-amyloid (Aβ) antibody with unique aβ binding properties promotes neuroprotection and glial engulfment of Aβ.
Adolfsson O, Pihlgren M, Toni N, Varisco Y, Buccarello AL, Antoniello K, Lohmann S, Piorkowska K, Gafner V, Atwal JK, Maloney J, Chen M, Gogineni A, Weimer RM, Mortensen DL, Friesenhahn M, Ho C, Paul R, Pfeifer A, Muhs A, Watts RJ. Adolfsson O, et al. J Neurosci. 2012 Jul 11;32(28):9677-89. doi: 10.1523/JNEUROSCI.4742-11.2012. J Neurosci. 2012. PMID: 22787053 Free PMC article. Clinical Trial. - Monitoring protein aggregation and toxicity in Alzheimer's disease mouse models using in vivo imaging.
Spires-Jones TL, de Calignon A, Meyer-Luehmann M, Bacskai BJ, Hyman BT. Spires-Jones TL, et al. Methods. 2011 Mar;53(3):201-7. doi: 10.1016/j.ymeth.2010.12.009. Epub 2010 Dec 14. Methods. 2011. PMID: 21163350 Free PMC article. - Targeting of nonlipidated, aggregated apoE with antibodies inhibits amyloid accumulation.
Liao F, Li A, Xiong M, Bien-Ly N, Jiang H, Zhang Y, Finn MB, Hoyle R, Keyser J, Lefton KB, Robinson GO, Serrano JR, Silverman AP, Guo JL, Getz J, Henne K, Leyns CE, Gallardo G, Ulrich JD, Sullivan PM, Lerner EP, Hudry E, Sweeney ZK, Dennis MS, Hyman BT, Watts RJ, Holtzman DM. Liao F, et al. J Clin Invest. 2018 May 1;128(5):2144-2155. doi: 10.1172/JCI96429. Epub 2018 Mar 30. J Clin Invest. 2018. PMID: 29600961 Free PMC article. - β-Hydroxybutyrate inhibits inflammasome activation to attenuate Alzheimer's disease pathology.
Shippy DC, Wilhelm C, Viharkumar PA, Raife TJ, Ulland TK. Shippy DC, et al. J Neuroinflammation. 2020 Sep 21;17(1):280. doi: 10.1186/s12974-020-01948-5. J Neuroinflammation. 2020. PMID: 32958021 Free PMC article.
References
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. - PubMed
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. - PMC - PubMed
- Bacskai BJ, Kajdasz ST, Christie RH, Carter C, Games D, Seubert P, Schenk D, Hyman BT. Imaging of amyloid-β deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nat Med. 2001;7:369–372. - PubMed
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, et al. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 NS057105/NS/NINDS NIH HHS/United States
- F33 AG029044-01/AG/NIA NIH HHS/United States
- P30 NS057105-03/NS/NINDS NIH HHS/United States
- P01 NS032636/NS/NINDS NIH HHS/United States
- NS32636/NS/NINDS NIH HHS/United States
- F32 AG029044/AG/NIA NIH HHS/United States
- F32 AG029044-03/AG/NIA NIH HHS/United States
- P01 NS032636-06A19005/NS/NINDS NIH HHS/United States
- F32 AG029044-02/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases