Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release - PubMed (original) (raw)
. 2008 Dec 24;28(52):14311-9.
doi: 10.1523/JNEUROSCI.2058-08.2008.
Luciano Minuzzi, Ruth M Krebs, David Elmenhorst, Markus Lang, Oliver H Winz, Constanze I Seidenbecher, Heinz H Coenen, Hans-Jochen Heinze, Karl Zilles, Emrah Düzel, Andreas Bauer
Affiliations
- PMID: 19109512
- PMCID: PMC6671462
- DOI: 10.1523/JNEUROSCI.2058-08.2008
Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release
Björn H Schott et al. J Neurosci. 2008.
Abstract
The dopaminergic mechanisms that control reward-motivated behavior are the subject of intense study, but it is yet unclear how, in humans, neural activity in mesolimbic reward-circuitry and its functional neuroimaging correlates are related to dopamine release. To address this question, we obtained functional magnetic resonance imaging (fMRI) measures of reward-related neural activity and [(11)C]raclopride positron emission tomography measures of dopamine release in the same human participants, while they performed a delayed monetary incentive task. Across the cohort, a positive correlation emerged between neural activity of the substantia nigra/ventral tegmental area (SN/VTA), the main origin of dopaminergic neurotransmission, during reward anticipation and reward-related [(11)C]raclopride displacement as an index of dopamine release in the ventral striatum, major target of SN/VTA dopamine neurons. Neural activity in the ventral striatum/nucleus accumbens itself also correlated with ventral striatal dopamine release. Additionally, high-reward-related dopamine release was associated with increased activation of limbic structures, such as the amygdala and the hippocampus. The observed correlations of reward-related mesolimbic fMRI activation and dopamine release provide evidence that dopaminergic neurotransmission plays a quantitative role in human mesolimbic reward processing. Moreover, the combined neurochemical and hemodynamic imaging approach used here opens up new perspectives for the investigation of molecular mechanisms underlying human cognition.
Figures
Figure 1.
Schematic illustration of the experimental paradigm. Top, Both the PET and the fMRI experiment consisted of a rewarded (135 rewarded trials, 45 neutral trials) and a neutral condition (135 neutral trials and 45 bogus reward trials), which were conducted on 2 consecutive days, counterbalanced across participants. The “reward” trials in the neutral condition consisted of reward-predicting cue pictures, followed by a neutral feedback. Bottom, The trials consisted of a cue picture indicating the possibility of a reward (indoor or outdoor scenes, counterbalanced across participants), followed by a target number and a feedback after a variable delay ranging from 1000 to 6000 ms. The feedback was positive or negative in the rewarded trials (arrow up or down) and neutral (“?”) in the neutral trials.
Figure 2.
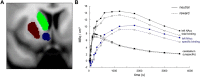
Reward-related [11C]raclopride displacement. A, Coronal section of the striatal ROIs in the left hemisphere. The corresponding ROIs in the right hemisphere were also segmented. B, Time–activity curves of a representative single subject. Total binding in the NAcc, unspecific binding in the cerebellum, and specific binding in the NAcc as the difference NAcc-cerebellum are shown. The _x_-axis depicts the time of PET scanning, starting with the injection of [11C]raclopride. The _y_-axis displays local radioactivity in the NAcc and cerebellum and the difference of NAcc and cerebellum activity, in MBq/mm3.
Figure 3.
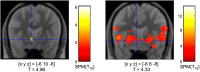
Dopamine release and fMRI activations in the ventral striatum (results from the voxel-based analysis). Left, [11C]raclopride displacement in the left nucleus accumbens. Right, Activation of the ventral striatum during reward anticipation in the fMRI study. Coordinates are given in MNI space; p < 0.005, uncorrected; extent threshold k = 15 voxels.
Figure 4.
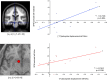
Correlation of dopamine release and fMRI activations. Top, Left, Location of the ROI for the left midbrain. Right, Across the study cohort, fMRI response in the left midbrain (segmented area) during reward anticipation was significantly correlated with [11C]raclopride displacement in the rewarded relative to the neutral condition. Bottom, Left, Representative ROI from a single subject. Six-millimeter spheres were centered at the local maxima of the reward anticipation response closest to [_x y z_] = [−6 10 −6] (the coordinate of maximal reward-related BPND decrease in PET), individually for each subject. Right, A significant correlation was observed between [11C]raclopride displacement and the fMRI response in the left nucleus accumbens.
Similar articles
- Personality traits are differentially associated with patterns of reward and novelty processing in the human substantia nigra/ventral tegmental area.
Krebs RM, Schott BH, Düzel E. Krebs RM, et al. Biol Psychiatry. 2009 Jan 15;65(2):103-10. doi: 10.1016/j.biopsych.2008.08.019. Epub 2008 Oct 2. Biol Psychiatry. 2009. PMID: 18835480 - Novelty increases the mesolimbic functional connectivity of the substantia nigra/ventral tegmental area (SN/VTA) during reward anticipation: Evidence from high-resolution fMRI.
Krebs RM, Heipertz D, Schuetze H, Duzel E. Krebs RM, et al. Neuroimage. 2011 Sep 15;58(2):647-55. doi: 10.1016/j.neuroimage.2011.06.038. Epub 2011 Jun 24. Neuroimage. 2011. PMID: 21723396 - Imaging human reward processing with positron emission tomography and functional magnetic resonance imaging.
Urban NB, Slifstein M, Meda S, Xu X, Ayoub R, Medina O, Pearlson GD, Krystal JH, Abi-Dargham A. Urban NB, et al. Psychopharmacology (Berl). 2012 May;221(1):67-77. doi: 10.1007/s00213-011-2543-6. Epub 2011 Nov 4. Psychopharmacology (Berl). 2012. PMID: 22052081 - Involvement of basal ganglia and orbitofrontal cortex in goal-directed behavior.
Hollerman JR, Tremblay L, Schultz W. Hollerman JR, et al. Prog Brain Res. 2000;126:193-215. doi: 10.1016/S0079-6123(00)26015-9. Prog Brain Res. 2000. PMID: 11105648 Review. - Cortico-Basal Ganglia reward network: microcircuitry.
Sesack SR, Grace AA. Sesack SR, et al. Neuropsychopharmacology. 2010 Jan;35(1):27-47. doi: 10.1038/npp.2009.93. Neuropsychopharmacology. 2010. PMID: 19675534 Free PMC article. Review.
Cited by
- Neurovascular coupling to D2/D3 dopamine receptor occupancy using simultaneous PET/functional MRI.
Sander CY, Hooker JM, Catana C, Normandin MD, Alpert NM, Knudsen GM, Vanduffel W, Rosen BR, Mandeville JB. Sander CY, et al. Proc Natl Acad Sci U S A. 2013 Jul 2;110(27):11169-74. doi: 10.1073/pnas.1220512110. Epub 2013 May 30. Proc Natl Acad Sci U S A. 2013. PMID: 23723346 Free PMC article. - Identifying predictors, moderators, and mediators of antidepressant response in major depressive disorder: neuroimaging approaches.
Phillips ML, Chase HW, Sheline YI, Etkin A, Almeida JR, Deckersbach T, Trivedi MH. Phillips ML, et al. Am J Psychiatry. 2015 Feb 1;172(2):124-38. doi: 10.1176/appi.ajp.2014.14010076. Am J Psychiatry. 2015. PMID: 25640931 Free PMC article. Review. - Dopaminergic involvement during mental fatigue in health and cocaine addiction.
Moeller SJ, Tomasi D, Honorio J, Volkow ND, Goldstein RZ. Moeller SJ, et al. Transl Psychiatry. 2012 Oct 23;2(10):e176. doi: 10.1038/tp.2012.110. Transl Psychiatry. 2012. PMID: 23092980 Free PMC article. - Motivational salience modulates hippocampal repetition suppression and functional connectivity in humans.
Zweynert S, Pade JP, Wüstenberg T, Sterzer P, Walter H, Seidenbecher CI, Richardson-Klavehn A, Düzel E, Schott BH. Zweynert S, et al. Front Hum Neurosci. 2011 Nov 17;5:144. doi: 10.3389/fnhum.2011.00144. eCollection 2011. Front Hum Neurosci. 2011. PMID: 22125521 Free PMC article. - The effect of risperidone on reward-related brain activity is robust to drug-induced vascular changes.
Hawkins PCT, Zelaya FO, O'Daly O, Holiga S, Dukart J, Umbricht D, Mehta MA. Hawkins PCT, et al. Hum Brain Mapp. 2021 Jun 15;42(9):2766-2777. doi: 10.1002/hbm.25400. Epub 2021 Mar 5. Hum Brain Mapp. 2021. PMID: 33666305 Free PMC article. Clinical Trial.
References
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. - PubMed
- Ahsan RL, Allom R, Gousias IS, Habib H, Turkheimer FE, Free S, Lemieux L, Myers R, Duncan JS, Brooks DJ, Koepp MJ, Hammers A. Volumes, spatial extents and a probabilistic atlas of the human basal ganglia and thalamus. Neuroimage. 2007;38:261–270. - PubMed
- Björklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. - PubMed
- Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci U S A. 1997;94:2569–2574. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical