Molecular basis of metastasis - PubMed (original) (raw)
Review
Molecular basis of metastasis
Anne C Chiang et al. N Engl J Med. 2008.
Abstract
Metastasis is the end product of an evolutionary process in which diverse interactions between cancer cells and their microenvironment yield alterations that allow these cells to transcend their programmed behavior. Tumor cells thus populate and flourish in new tissue habitats and, ultimately, cause organ dysfunction and death. Understanding the many molecular players and processes involved in metastasis could lead to effective, targeted approaches to prevent and treat cancer metastasis.
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures
Figure 1. Tumor Initiation and Metastasis
The initiation and progression of tumors depend on the acquisition of specific functions by cancer cells at both the primary and metastatic sites. Functions associated with tumor initiation are provided by oncogenic mutations and inactivation of tumor-suppressor genes. Functions associated with the initiation of metastasis include functions to which tumor cells resort for local invasion and for circumventing hypoxia and other limitations facing a growing tumor. Most functions for the initiation of both the tumor and metastasis remain essential for cancer cells to continue their metastatic development. Functions for metastasis progression provide a local advantage in a primary tumor and a distinct and sometimes organ-specific function during metastasis. Cancer cells that are endowed with these three sets of functions still depend on functions associated with metastasis virulence; these functions confer a selective advantage solely during the adaptation and takeover of a specific organ microenvironment. Genes associated with each of these functions have been identified in recent years.
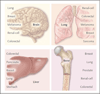
Figure 2. Patterns of Metastatic Spread of Solid Tumors
Brain metastases may occur as a result of hematogenous spread late in the course of a widely metastatic tumor, or as a result of secondary metastasis from a primary or a metastatic tumor that can access the arterial circulation through the pulmonary venous circulation to seed the brain. Tumors with the highest incidence of brain metastases include lung carcinoma, breast carcinoma, melanoma, and to a lesser extent, renal-cell and colorectal carcinomas. Leptomeningeal disease may develop through the spread of cancer cells through perineural lymphatic vessels, and it is a sign of advanced disease. Some tumors have a strong proclivity for dissemination to the lungs; for example, in one study, the rate of dissemination associated with sarcoma was 23%. Other tumors that frequently spread to the lungs include renal-cell, colorectal, melanoma, and breast carcinomas., Gastrointestinal tumors easily access the liver circulation through the portal-vein system. The incidence of liver metastases is highest among patients with colorectal or pancreatic cancer, followed by breast and lung cancers. Estrogen-receptor–negative breast-cancer tumors more often metastasize to visceral organs, including the liver, whereas estrogen-receptor–positive breast cancer more often metastasizes to the bone. Bone metastasis occurs in patients with primary tumors associated with breast, lung, prostate, renal-cell, and colon cancer, in this order of frequency. Bone metastases may be primarily osteolytic or osteoblastic, depending on the tumor of origin.
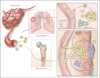
Figure 3. Genes, Functions, and Cellular Players in Organ-Specific Metastasis
Organ-specific metastasis of breast-cancer cells involves different molecular players during colonization of the lungs and the bones. In the lung, cancer cells producing EREG, COX-2, MMP-1, and ANGPTL4 are better equipped to exit the pulmonary vasculature, since these factors alter the integrity of lung microcapillary endothelia; this function is less important for infiltration into the bone marrow because of the naturally fenestrated structure of the bone marrow sinusoid vasculature. In the lung parenchyma, the activity of the antidifferentiation gene ID1 and interactions with still unknown “niche” factors promote tumor reinitiation. In the bone marrow, stromal-cell–derived factor 1 (SDF-1), acting through its chemokine (C-X-C motif) receptor (CXCR4) on cancer cells, is thought to provide cell-survival functions. The secretion of parathyroid hormone–related peptide (PTHrP), interleukin-6, tumor necrosis factor α (TNF-α), interleukin-11, and other factors by cancer cells stimulates osteoblasts to release the ligand for the receptor activator of nuclear factor-κB (RANKL), which in turn stimulates osteoclast differentiation from myeloid progenitor cells. Other cancer cell–derived factors suppress the production of the RANKL antagonist osteoprotegerin, augmenting the efficacy of RANKL. The lytic action of osteoclasts releases bone matrix–associated growth factors, including transforming growth factor β (TGF-β), insulin-like growth factor I (IGF-I), and bone morphogenetic proteins (BMPs). IGF-I is a survival factor, and TGF-β incites cancer cells to further release PTHrP, interleukin-11, and other prometastatic factors, establishing a vicious cycle.
Comment in
- Molecular basis of metastasis.
Varki A, Varki NM, Borsig L. Varki A, et al. N Engl J Med. 2009 Apr 16;360(16):1678-9; author reply 1679-80. doi: 10.1056/NEJMc090143. N Engl J Med. 2009. PMID: 19369678 No abstract available. - Molecular basis of metastasis.
Batistatou A, Charalabopoulos A, Charalabopoulos K. Batistatou A, et al. N Engl J Med. 2009 Apr 16;360(16):1679; author reply 1679-80. N Engl J Med. 2009. PMID: 19373966 No abstract available. - Molecular basis of metastasis.
Lee JJ, Lotze MT. Lee JJ, et al. N Engl J Med. 2009 Apr 16;360(16):1679; author reply 1679-80. N Engl J Med. 2009. PMID: 19373967 No abstract available.
Similar articles
- Tumor metastasis: mechanistic insights and clinical challenges.
Steeg PS. Steeg PS. Nat Med. 2006 Aug;12(8):895-904. doi: 10.1038/nm1469. Nat Med. 2006. PMID: 16892035 Review. No abstract available. - [Research on the antimetastatic agents].
Takeshima H. Takeshima H. Nihon Rinsho. 2005 Sep;63 Suppl 9:79-85. Nihon Rinsho. 2005. PMID: 16201504 Review. Japanese. No abstract available. - Hypoxia, gene expression, and metastasis.
Chan DA, Giaccia AJ. Chan DA, et al. Cancer Metastasis Rev. 2007 Jun;26(2):333-9. doi: 10.1007/s10555-007-9063-1. Cancer Metastasis Rev. 2007. PMID: 17458506 Review. - Genes that regulate metastasis and angiogenesis.
Webb CP, Vande Woude GF. Webb CP, et al. J Neurooncol. 2000 Oct-Nov;50(1-2):71-87. doi: 10.1023/a:1006466605356. J Neurooncol. 2000. PMID: 11245283 Review. - Mechanisms of metastasis: seed and soil.
Piris A, Mihm MC Jr. Piris A, et al. Cancer Treat Res. 2007;135:119-27. doi: 10.1007/978-0-387-69219-7_9. Cancer Treat Res. 2007. PMID: 17953412 Review. No abstract available.
Cited by
- Matricellular proteins: a sticky affair with cancers.
Chong HC, Tan CK, Huang RL, Tan NS. Chong HC, et al. J Oncol. 2012;2012:351089. doi: 10.1155/2012/351089. Epub 2012 Feb 9. J Oncol. 2012. PMID: 22481923 Free PMC article. - Less is more: low expression of MT1-MMP is optimal to promote migration and tumourigenesis of breast cancer cells.
Cepeda MA, Pelling JJ, Evered CL, Williams KC, Freedman Z, Stan I, Willson JA, Leong HS, Damjanovski S. Cepeda MA, et al. Mol Cancer. 2016 Oct 18;15(1):65. doi: 10.1186/s12943-016-0547-x. Mol Cancer. 2016. PMID: 27756325 Free PMC article. - Characteristic Mutational Damages in Gastric and Colorectal Adenocarcinomas.
Yermekova S, Orazgaliyeva M, Goncharova T, Rakhimbekova F, Kaidarova D, Shatkovskaya O. Yermekova S, et al. Asian Pac J Cancer Prev. 2023 Nov 1;24(11):3939-3947. doi: 10.31557/APJCP.2023.24.11.3939. Asian Pac J Cancer Prev. 2023. PMID: 38019254 Free PMC article. - Molecular mechanisms and clinical management of cancer bone metastasis.
Wang M, Xia F, Wei Y, Wei X. Wang M, et al. Bone Res. 2020 Jul 29;8(1):30. doi: 10.1038/s41413-020-00105-1. eCollection 2020. Bone Res. 2020. PMID: 32793401 Free PMC article. Review. - Pharmaceutical Advances and Proteomics Researches.
KhalKhal E, Rezaei-Tavirani M, Rostamii-Nejad M. KhalKhal E, et al. Iran J Pharm Res. 2019 Fall;18(Suppl1):51-67. doi: 10.22037/ijpr.2020.112440.13758. Iran J Pharm Res. 2019. PMID: 32802089 Free PMC article. Review.
References
- DeVita VT Jr, Lawrence TS, Rosenberg SA, editors. DeVita, Hellman, and Rosenberg’s Cancer: principles & practice of oncology. 8th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2008.
- Weinberg RA. The biology of cancer. New York: Garland Science; 2007.
- Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. - PubMed
- Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. - PubMed
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources