Regulation of LKB1/STRAD localization and function by E-cadherin - PubMed (original) (raw)
Regulation of LKB1/STRAD localization and function by E-cadherin
Michael Sebbagh et al. Curr Biol. 2009.
Abstract
LKB1 kinase is a tumor suppressor that is causally linked to Peutz-Jeghers syndrome. In complex with the pseudokinase STRAD and the scaffolding protein MO25, LKB1 phosphorylates and activates AMPK family kinases, which mediate many cellular processes. The prototypical family member AMPK regulates cell energy metabolism and epithelial apicobasal polarity. This latter event is also dependent on E-cadherin-mediated adherens junctions (AJs) at lateral borders. Strikingly, overexpression of LKB1/STRAD can also trigger establishment of epithelial polarity in the absence of cell-cell or cell-matrix contacts. However, the upstream factors that normally govern LKB1/STRAD function are unknown. Here we show by immunostaining and fluorescence resonance energy transfer that active LKB1/STRAD kinase complex colocalizes with E-cadherin at AJs. LKB1/STRAD localization and AMPK phosphorylation require E-cadherin-dependent maturation of AJs. However, LKB1/STRAD complex kinase activity is E-cadherin independent. These data suggest that in polarized epithelial cells, E-cadherin regulates AMPK phosphorylation by controlling the localization of the LKB1 complex. The LKB1 complex therefore appears to function downstream of E-cadherin in tumor suppression.
Figures
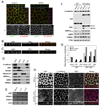
Figure 1. LKB1 localizes at adherens junction level in polarized epithelial cells
(A) Polarized Caco-2 stained for LKB1 (green) and E-cadherin (red). (B) xz projection of polarized Caco-2 cells stained for LKB1 (green) and villin (red) or (C) for LKB1 (green) and ZO-1 (red). (D) Polarized Caco-2 cells were fractionated as described in “experimental procedures” and analyzed for indicated proteins. Results are representative of two independent experiments. (E) MDCK clones stably knocked down for LKB1 (cl) by shRNA or control construct (shLuc) transiently transfected by either GFP alone or GFP-LKB1wt were analysed by Western blotting for indicated proteins. (F) AMPKp-172/total AMPK ratio (black bar) were determined and plotted with endogenous (endo, white bar) and exogenous (exo, grey bar) LKB1, all relative to control. Values are means ± S.D., (n=2). (G) Polarized MDCK shLuc and shLKB1 cl 14 were stained for LKB1 (green), E-cadherin (purple) and villin (red). Bars, 10µm.
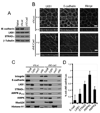
Figure 2. STRAD localizes with LKB1 at adherens junction level
(A) Polarized MDCK shLuc or shLKB1 cl 14 cells were stained for LKB1 (green), E-cadherin (purple) and STRAD (red). (B) Polarized Caco-2 clone knocked down for LKB1 (cl A12) or not (shLuc) were fractionated as in figure 1. (C) Membranous shLKB1 cl A12/shLuc ratio for LKB1, STRADα, total or phosphorylated AMPK, normalized to E-cadherin. Values are means ± S.D., (n=3). (D) MDCK cells on fibronectin-coated coverslips were transiently transfected with YFP-STRADα (yellow) plus either CFP-Myr, wild type or SL26 CFP-LKB1 (blue). FRET was measured as described in “Experimental Procedures”. The asterisks mark an unidentified perinuclear compartment. (E) Projections of FRET images from CFP-LKB1-WT and YFP-STRADα. FRET intensity is displayed on a pseudocolor scale in arbitrary units. Bars, 10µm.
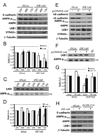
Figure 3. E-cadherin is required to localize LKB1 at adherens junctions level
(A) MDCK cells expressing control (shLuc) or E-cadherin (shE-cad) shRNA vectors were analysed by Western blot for the indicating proteins. (B) Cells were stained for LKB1 (green) and E-cadherin (red). Bars, 10µm. (C) MDCK cells described in A were fractionated as described in figure 1, (D) Ratio of membranous shE-cad/shLuc of LKB1, STRADα, total or phosphorylated AMPK, normalized to integrin. Values are means ± S.D., (n=2).
Figure 4. E-cadherin knockdown decrease AMPK activation
(A) MDCK cells were transiently transfected with control (shLuc) or shRNA targetting canine E-cadherin (shE-cad). Cells were extracted at indicated times and analyzed by Western blot for indicated proteins. (B) T172 AMPK phosphorylation and E-cadherin levels were quantified and normalized to total AMPK. Values are percent relative to levels in shLuc or shE-cad cells at 24h (means ± S.D. n=3). pAMPK: white bars; E-cadherin: black bars. (C) The same lysates were immunoprecipitated with anti-LKB1 and kinase activity analyzed in vitro (IVK). (D) Kinase activity was quantified and normalized to total immunoprecipitated LKB1, plotted as described in B (means ± S.D. n=4). Control (shLuc) and E-cadherin-depleted MDCK cells were transiently transfected or not with wild type human E-cadherin (pcDNAhE-cad). After 24h cells, were extracted and treated as in A and C (E, F respectively**)** and quantified (G). T172 AMPK phosphorylation in cells (white bar), LKB1 IP kinase activity (TK) (black bar), control shLuc cells mock transfected was used as reference (means ± S.D. n=3). (H) Stable control (shLuc) and LKB1 depleted (shLKB cl14) MDCK cells were transiently transfected or not with shRNA targeting canine E-cadherin (shE-cad). After 72h, cells were lysed and analyzed by Western blotting with the specified antibodies (n=2).
Similar articles
- Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade.
Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Mäkelä TP, Alessi DR, Hardie DG. Hawley SA, et al. J Biol. 2003;2(4):28. doi: 10.1186/1475-4924-2-28. Epub 2003 Sep 24. J Biol. 2003. PMID: 14511394 Free PMC article. - Analysis of the LKB1-STRAD-MO25 complex.
Boudeau J, Scott JW, Resta N, Deak M, Kieloch A, Komander D, Hardie DG, Prescott AR, van Aalten DM, Alessi DR. Boudeau J, et al. J Cell Sci. 2004 Dec 15;117(Pt 26):6365-75. doi: 10.1242/jcs.01571. Epub 2004 Nov 23. J Cell Sci. 2004. PMID: 15561763 - Activation of the tumour suppressor kinase LKB1 by the STE20-like pseudokinase STRAD.
Baas AF, Boudeau J, Sapkota GP, Smit L, Medema R, Morrice NA, Alessi DR, Clevers HC. Baas AF, et al. EMBO J. 2003 Jun 16;22(12):3062-72. doi: 10.1093/emboj/cdg292. EMBO J. 2003. PMID: 12805220 Free PMC article. - LKB1 biology: assessing the therapeutic relevancy of LKB1 inhibitors.
Trelford CB, Shepherd TG. Trelford CB, et al. Cell Commun Signal. 2024 Jun 6;22(1):310. doi: 10.1186/s12964-024-01689-5. Cell Commun Signal. 2024. PMID: 38844908 Free PMC article. Review. - LKB1-dependent signaling pathways.
Alessi DR, Sakamoto K, Bayascas JR. Alessi DR, et al. Annu Rev Biochem. 2006;75:137-63. doi: 10.1146/annurev.biochem.75.103004.142702. Annu Rev Biochem. 2006. PMID: 16756488 Review.
Cited by
- Primary cilia regulate mTORC1 activity and cell size through Lkb1.
Boehlke C, Kotsis F, Patel V, Braeg S, Voelker H, Bredt S, Beyer T, Janusch H, Hamann C, Gödel M, Müller K, Herbst M, Hornung M, Doerken M, Köttgen M, Nitschke R, Igarashi P, Walz G, Kuehn EW. Boehlke C, et al. Nat Cell Biol. 2010 Nov;12(11):1115-22. doi: 10.1038/ncb2117. Epub 2010 Oct 24. Nat Cell Biol. 2010. PMID: 20972424 Free PMC article. - Controlling the master-upstream regulation of the tumor suppressor LKB1.
Kullmann L, Krahn MP. Kullmann L, et al. Oncogene. 2018 Jun;37(23):3045-3057. doi: 10.1038/s41388-018-0145-z. Epub 2018 Mar 15. Oncogene. 2018. PMID: 29540834 Review. - Folliculin controls lung alveolar enlargement and epithelial cell survival through E-cadherin, LKB1, and AMPK.
Goncharova EA, Goncharov DA, James ML, Atochina-Vasserman EN, Stepanova V, Hong SB, Li H, Gonzales L, Baba M, Linehan WM, Gow AJ, Margulies S, Guttentag S, Schmidt LS, Krymskaya VP. Goncharova EA, et al. Cell Rep. 2014 Apr 24;7(2):412-423. doi: 10.1016/j.celrep.2014.03.025. Epub 2014 Apr 13. Cell Rep. 2014. PMID: 24726356 Free PMC article. - It takes energy to resist force.
Bays JL, DeMali KA. Bays JL, et al. Cell Cycle. 2017 Oct 2;16(19):1733-1734. doi: 10.1080/15384101.2017.1360654. Epub 2017 Aug 18. Cell Cycle. 2017. PMID: 28820336 Free PMC article. No abstract available. - Hepatocyte polarity.
Treyer A, Müsch A. Treyer A, et al. Compr Physiol. 2013 Jan;3(1):243-87. doi: 10.1002/cphy.c120009. Compr Physiol. 2013. PMID: 23720287 Free PMC article. Review.
References
- Sanchez-Cespedes M. A role for LKB1 gene in human cancer beyond the Peutz-Jeghers syndrome. Oncogene. 2007;26:7825–7832. - PubMed
- Luo Z, Saha AK, Xiang X, Ruderman NB. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci. 2005;26:69–76. - PubMed
- Lee JH, Koh H, Kim M, Kim Y, Lee SY, Karess RE, Lee SH, Shong M, Kim JM, Kim J, Chung J. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature. 2007;447:1017–1020. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases