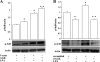Role of acylglycerol kinase in LPA-induced IL-8 secretion and transactivation of epidermal growth factor-receptor in human bronchial epithelial cells - PubMed (original) (raw)
Role of acylglycerol kinase in LPA-induced IL-8 secretion and transactivation of epidermal growth factor-receptor in human bronchial epithelial cells
Satish Kalari et al. Am J Physiol Lung Cell Mol Physiol. 2009 Mar.
Abstract
LPA (lysophosphatidic acid) is a potent bioactive phospholipid, which regulates a number of diverse cellular responses through G protein-coupled LPA receptors. Intracellular LPA is generated by the phosphorylation of monoacylglycerol by acylglycerol kinase (AGK); however, the role of intracellular LPA in signaling and cellular responses remains to be elucidated. Here, we investigated signaling pathways of IL-8 secretion mediated by AGK and intracellular LPA in human bronchial epithelial cells (HBEpCs). Expression of AGK in HBEpCs was detected by real-time PCR, and overexpressed AGK was mainly localized in mitochondria as determined by immunofluorescence and confocal microscopy. Overexpression of lentiviral AGK wild type increased intracellular LPA production ( approximately 1.8-fold), enhanced LPA-mediated IL-8 secretion, and stimulated tyrosine phosphorylation epidermal growth factor-receptor (EGF-R). Furthermore, downregulation of native AGK by AGK small interfering RNA decreased intracellular LPA levels ( approximately 2-fold) and attenuated LPA-induced p38 MAPK, JNK, and NF-kappaB activation, tyrosine phosphorylation of EGF-R, and IL-8 secretion. These results suggest that native AGK regulates LPA-mediated IL-8 secretion involving MAPKs, NF-kappaB, and transactivation of EGF-R. Thus AGK may play an important role in innate immunity and airway remodeling during inflammation.
Figures
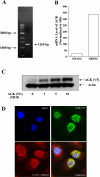
Fig. 1.
Overexpressed acylglycerol kinase (AGK) is localized to mitochondria. A: total RNA extracted from human bronchial epithelial cells (HBEpCs) and transcription of the gene encoding AGK were assessed by RT-PCR with AGK specific cloning primers. Left lane contains molecular size markers, and AGK amplification is indicated by arrows. B: RT-PCR analysis of AGK from total RNA extracted from both HBEpCs and HPAECs was performed by real-time PCR using AGK specific primers as described in
materials and methods
. Relative expression of AGK was normalized to 18S RNA. C: HBEpCs (∼70% confluence in 35-mm dishes) were infected with vector control or V5-tagged lentiviral AGK wild type [1, 5, and 10 multiplicities of infection (MOI)] in complete BEBM for 48 h. Cell lysates were prepared as described in
materials and methods
and subjected to SDS-PAGE and Western blotting with anti-V5 and anti-actin antibodies. D: HBEpCs grown on coverslips to ∼70% confluence were infected with V5-tagged lentiviral AGK wild type (5 MOI) for 48 h. Cells were immunostained with anti-V5 antibody and coimmunostained with MitoTracker Red CMXRos, followed by confocal microscopy. The confocal image is representative of 3 independent experiments.
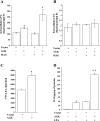
Fig. 2.
Effect of AGK overexpression on lysophosphatidic acid (LPA) synthesis and IL-8 secretion. HBEpCs were seeded in 6-well dishes and infected with vector (lenti LacZ) or V5-tagged lentiviral AGK wild type for 48 h. Media were aspirated, and 1 μM monoacylglycerol (MAG), in basal media without any growth factors, was added to cells and incubated for an additional 1 h. Lipids were extracted from both cells and media for LPA measurements as described in
materials and methods
. A: intracellular (cells) analysis of LPA accumulation compared between vector and V5-tagged lentiviral AGK wild type infected cells, either in presence or absence of MAG, by LC-MS/MS. B: extracellular media were analyzed for LPA accumulation in vector and V5-tagged lentiviral AGK wild type transfected cells, either in presence or absence of MAG, by LC-MS/MS. C: HBEpCs were seeded in 6-well dishes and infected with vector control or V5-tagged AGK wild type for 48 h and incubated for 3 h with 50 μCi/ml [32P]orthophosphate. Labeled LPA generated in cells was quantified after separation of the lipid extracts by thin-layer chromatography and scintillation counting as described in
materials and methods
. Values are means ± SD from 3 independent experiments. *Significantly different from vector control cells at P < 0.05. D: following treatment with 1 μM LPA for 3 h, media were collected from cells infected with either vector control or V5-tagged lentiviral AGK wild type, centrifuged to remove any floating cells, and analyzed for IL-8 release by ELISA. Values are means ± SD of at least 3 independent experiments. *Significantly different from controls at P < 0.05. **Significantly different from LPA-treated cells at P < 0.001.
Fig. 3.
Effect of AGK small interfering RNA (siRNA) on LPA synthesis and IL-8 secretion. HBEpCs in 6-well dishes were transfected with either scrambled siRNA or AGK siRNA (10 nM) for 48 h. A: RT-PCR analysis of AGK message from total RNA extracted from HBEpCs was analyzed by real-time PCR using AGK specific primers, as described in
materials and methods
. Relative expression of AGK was normalized to 18S RNA. B: lipids were extracted from scrambled or AGK siRNA transfected cells, and LPA was measured by LC-MS/MS as described in
materials and methods
. C: cells were transfected with scrambled or AGK siRNA before LPA (1 μM) challenge for 3 h, centrifuged to remove any floating cells, and analyzed for released IL-8 by ELISA. Values are means ± SD of at least 3 independent experiments. *Significantly different from controls at P < 0.05. **Significantly different from cells not challenged with LPA at P < 0.001. ***Significantly different from LPA-treated cells at P < 0.01.
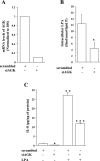
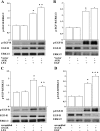
Fig. 4.
Role of MAPK in AGK-mediated IL-8 secretion. HBEpCs grown in 6-well dishes to ∼95% confluence were infected with either vector control or V5-tagged lentiviral AGK wild type for 48 h or transfected with scrambled siRNA or AGK siRNA (10 nM) for 48 h before LPA (1 μM) challenge. In A and B, cells were exposed to LPA for 10 min, and cell lysates (20 μg proteins) were subjected to SDS-PAGE and Western blotted with phospho-p38 and total p38 (A) and phospho-JNK and total JNK antibodies (B). Blots were scanned and analyzed by image analyzer, and increase in phosphorylation was normalized with total p38 MAPK or total JNK. Shown are representative blots, and values are means ± SD from 3 independent experiments. *Significantly different from controls at P < 0.01. **Significantly different from LPA-treated cells at P < 0.05. In C and D, HBEpCs grown in 6-well dishes to ∼60% confluence were transfected with scrambled siRNA or AGK siRNA for 48 h and then challenged with LPA (1 μM) for 10 min. Cell lysates (20 μg proteins) were subjected to SDS-PAGE and Western blotted with phospho-p38 and total p38 (C) and phospho-JNK and total JNK antibodies (D). Shown are representative blots, and values are means ± SD from 3 independent experiments. *Significantly different from controls at P < 0.01. **Significantly different from LPA-treated cells at P < 0.005.
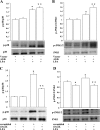
Fig. 5.
Role of AGK in LPA-dependent activation of NF-κB. In A, HBEpCs grown in 6-well dishes to ∼95% confluence were infected with either vector control or V5-tagged lentiviral AGK wild type for 48 h and then challenged with medium alone or medium containing LPA (1 μM) for 10 min. Cell lysates (20 μg proteins) were subjected to SDS-PAGE and Western blotted with phospho-IκB and actin antibodies. Shown are representative blots, and values are means ± SD from 3 independent experiments normalized to total actin. *Significantly different from controls at P < 0.01. **Significantly different from LPA-treated cells at P < 0.05. In B, HBEpCs grown in 6-well dishes to ∼60% confluence were transfected with scrambled siRNA or AGK siRNA (10 nM) for 48 h and then challenged with LPA (1 μM) for 10 min. Cell lysates (20 μg proteins) were subjected to SDS-PAGE and Western blotted with phospho-IκB and anti-actin antibodies. Shown are representative blots, and values are means ± SD from 3 independent experiments normalized to total actin. *Significantly different from controls at P < 0.01. **Significantly different from LPA-treated cells at P < 0.005.
Fig. 6.
Role of AGK in EGF-R transactivation by LPA. In A and B, HBEpCs grown in 6-well dishes to ∼95% confluence were infected with either vector control or V5-tagged lentiviral AGK wild type for 48 h and then challenged with either LPA (1 μM) or EGF (20 ng/ml) for 15 min. Cell lysates (20 μg proteins) were subjected to SDS-PAGE and Western blotted with phospho-EGF-R, total EGF-R, or total ERK1/2 antibodies. Shown are representative blots, and values are means ± SD from 3 independent experiments normalized to total ERK. *Significantly different from controls at P < 0.005. **Significantly different from either LPA- or EGF-treated cells at P < 0.02. In C and D, HBEpCs grown in 6-well dishes to ∼60% confluence were transfected with scrambled siRNA or AGK siRNA (10 nM) for 48 h and then challenged with either LPA (1 μM) or EFG (20 ng/ml) for 15 min. Cell lysates (20 μg proteins) were subjected to SDS-PAGE and Western blotted with phospho-EGF-R, total EGF-R, or total ERK1/2 antibodies. Shown are representative blots, and values are means ± SD from 3 independent experiments normalized to total ERK. *Significantly different from controls at P < 0.01. **Significantly different from LPA-treated cells at P < 0.001. ***Significantly different from EGF-treated cells at P < 0.05.
Similar articles
- Transcriptional regulation of lysophosphatidic acid-induced interleukin-8 expression and secretion by p38 MAPK and JNK in human bronchial epithelial cells.
Saatian B, Zhao Y, He D, Georas SN, Watkins T, Spannhake EW, Natarajan V. Saatian B, et al. Biochem J. 2006 Feb 1;393(Pt 3):657-68. doi: 10.1042/BJ20050791. Biochem J. 2006. PMID: 16197369 Free PMC article. - Regulation of lysophosphatidic acid-induced epidermal growth factor receptor transactivation and interleukin-8 secretion in human bronchial epithelial cells by protein kinase Cdelta, Lyn kinase, and matrix metalloproteinases.
Zhao Y, He D, Saatian B, Watkins T, Spannhake EW, Pyne NJ, Natarajan V. Zhao Y, et al. J Biol Chem. 2006 Jul 14;281(28):19501-11. doi: 10.1074/jbc.M511224200. Epub 2006 May 10. J Biol Chem. 2006. PMID: 16687414 Free PMC article. - Lysophosphatidic acid signaling in airway epithelium: role in airway inflammation and remodeling.
Zhao Y, Natarajan V. Zhao Y, et al. Cell Signal. 2009 Mar;21(3):367-77. doi: 10.1016/j.cellsig.2008.10.010. Epub 2008 Oct 26. Cell Signal. 2009. PMID: 18996473 Free PMC article. Review. - Critical role of acylglycerol kinase in epidermal growth factor-induced mitogenesis of prostate cancer cells.
Spiegel S, Milstien S. Spiegel S, et al. Biochem Soc Trans. 2005 Dec;33(Pt 6):1362-5. doi: 10.1042/BST0331362. Biochem Soc Trans. 2005. PMID: 16246119 Review.
Cited by
- Acylglycerol kinase promotes cell proliferation and tumorigenicity in breast cancer via suppression of the FOXO1 transcription factor.
Wang X, Lin C, Zhao X, Liu A, Zhu J, Li X, Song L. Wang X, et al. Mol Cancer. 2014 May 8;13:106. doi: 10.1186/1476-4598-13-106. Mol Cancer. 2014. PMID: 24886245 Free PMC article. - Characterization of the properties of a selective, orally bioavailable autotaxin inhibitor in preclinical models of advanced stages of liver fibrosis.
Baader M, Bretschneider T, Broermann A, Rippmann JF, Stierstorfer B, Kuttruff CA, Mark M. Baader M, et al. Br J Pharmacol. 2018 Feb;175(4):693-707. doi: 10.1111/bph.14118. Epub 2018 Jan 17. Br J Pharmacol. 2018. PMID: 29197066 Free PMC article. - A novel AGK splicing mutation in a patient with Sengers syndrome and left ventricular non-compaction cardiomyopathy.
Fan P, Yang KQ, Han B, Kong D, Yin WH, Li JH, Yang ZX, Niu LL, Fu CS, Rong CZ, Lin YH, Wang H, Zhou XL, Gao LG, Qin XC, Tian T. Fan P, et al. Pediatr Res. 2023 Aug;94(2):683-690. doi: 10.1038/s41390-023-02515-3. Epub 2023 Feb 9. Pediatr Res. 2023. PMID: 36759750 - Lysophosphatidic acid increases soluble ST2 expression in mouse lung and human bronchial epithelial cells.
Zhao J, Chen Q, Li H, Myerburg M, Spannhake EW, Natarajan V, Zhao Y. Zhao J, et al. Cell Signal. 2012 Jan;24(1):77-85. doi: 10.1016/j.cellsig.2011.08.004. Epub 2011 Aug 17. Cell Signal. 2012. PMID: 21871564 Free PMC article. - Mechanisms of Lysophosphatidic Acid-Mediated Lymphangiogenesis in Prostate Cancer.
Wu PY, Lin YC, Huang YL, Chen WM, Chen CC, Lee H. Wu PY, et al. Cancers (Basel). 2018 Oct 31;10(11):413. doi: 10.3390/cancers10110413. Cancers (Basel). 2018. PMID: 30384405 Free PMC article. Review.
References
- Barekzi E, Roman J, Hise K, Georas S, Steinke JW. Lysophosphatidic acid stimulates inflammatory cascade in airway epithelial cells. Prostaglandins Leukot Essent Fatty Acids 74: 357–363, 2006. - PubMed
- Berdyshev EV, Gorshkova IA, Garcia JG, Natarajan V, Hubbard WC. Quantitative analysis of sphingoid base-1-phosphates as bisacetylated derivatives by liquid chromatography-tandem mass spectrometry. Anal Biochem 339: 129–136, 2005. - PubMed
- Berdyshev EV, Gorshkova IA, Usatyuk P, Zhao Y, Saatian B, Hubbard W, Natarajan V. De novo biosynthesis of dihydrosphingosine-1-phosphate by sphingosine kinase 1 in mammalian cells. Cell Signal 18: 1779–1792, 2006. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous