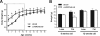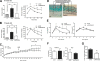Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI - PubMed (original) (raw)
Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI
Tayeba Khan et al. Mol Cell Biol. 2009 Mar.
Abstract
Adipocytes are embedded in a unique extracellular matrix whose main function is to provide mechanical support, in addition to participating in a variety of signaling events. During adipose tissue expansion, the extracellular matrix requires remodeling to accommodate adipocyte growth. Here, we demonstrate a general upregulation of several extracellular matrix components in adipose tissue in the diabetic state, therefore implicating "adipose tissue fibrosis" as a hallmark of metabolically challenged adipocytes. Collagen VI is a highly enriched extracellular matrix component of adipose tissue. The absence of collagen VI results in the uninhibited expansion of individual adipocytes and is paradoxically associated with substantial improvements in whole-body energy homeostasis, both with high-fat diet exposure and in the ob/ob background. Collectively, our data suggest that weakening the extracellular scaffold of adipocytes enables their stress-free expansion during states of positive energy balance, which is consequently associated with an improved inflammatory profile. Therefore, the disproportionate accumulation of extracellular matrix components in adipose tissue may not be merely an epiphenomenon of metabolically challenging conditions but may also directly contribute to a failure to expand adipose tissue mass during states of excess caloric intake.
Figures
FIG. 1.
Collagen VI expression levels correlate with different metabolic states. (A) Gene expression profiling of the major adipose tissue-expressed collagens in metabolically challenged mouse models was performed by microarray analysis. All microarray analyses were performed using epididymal fat. Results are expressed as the change in gene expression in db/db compared to WT mice (top graph), the change in WT mice treated with PPARγ agonist (30 mg/kg of COOH for 7 days) compared to WT mice treated with vehicle (middle graph), and the change in adiponectin-overexpressing mice compared to WT mice (bottom graph). Labeled cRNA from each individual experimental strain and treatment (n ≥ three mice) was competitively hybridized against a cRNA pool from the respective control group (n ≥ three mice) onto Agilent microarrays. A positive change is indicative of an upregulation in the experimental strain or treatment group, and vice versa. (B) Collagen VI is the predominantly expressed collagen in fat. The major adipose tissue-expressed collagens (collagen I, II, III, IV, V, and VI) were measured in epididymal, mesenteric, inguinal, and perirenal fat pads by qRT-RCR in 8-week old WT mice (n = 2). Expression levels were normalized to 18S rRNA. A representative example is shown. (C) Collagen VI expression is enriched in adipose tissue. Collagen VI alpha 3 levels were measured by qRT-PCR in the major fat pads and other tissues of 8-week-old WT mice (n = 2). Expression levels were normalized to 18S rRNA. A representative example is shown. (D) Collagen VI alpha 3 expression levels positively correlate to metabolically challenged states. Expression levels were determined by using Agilent microarrays, as described for panel A. Results show the change in collagen VI alpha 3 expression levels in ob/ob and db/db mice versus WT mice and the change in WT mice treated with 10 or 30 mg/kg (mpk) PPARγ agonist versus vehicle treatment (upper panel) (n ≥ three mice/group, except for the 10-mg/kg PPARγ agonist treatment, which included two mice). Collagen VI expression was reduced in adipose tissue by PPARγ treatment (middle and lower panels). Wild-type mice (10 weeks old) were treated with 30 mg/kg COOH or vehicle for 14 days by oral gavage. Collagen VI alpha 1, alpha 2, and alpha 3 mRNA levels were measured in epididymal (middle panel) and mesenteric (lower panel) fat by qRT-PCR and were normalized to 18S rRNA (mean ± standard error; n = four mice/group). (E) Collagen VI expression is increased in metabolically obese individuals. Collagen VI alpha 3 levels were measured in subcutaneous abdominal and gluteal (peripheral) adipose tissue in Asian Indians (18 men and 4 women), a metabolically obese group, and compared to control Caucasian populations of similar ages (27 ± 4 and 28 ± 7 years, respectively) and body mass indices (24 ± 4 and 25 ± 6 kg/m2, respectively). Expression levels were measured by qRT-PCR and normalized to 18S rRNA (means ± standard errors; n = 15 to 19 Caucasians, n = 24 to 30 Asians). *, P < 0.05; **, P < 0.01 by Student's t test.
FIG. 2.
col6KOob/ob mice have reduced body weights due to a decrease in fat mass at a young age. (A) Body weight was monitored for a period of 10 weeks (mean ± standard error; n = four mice/group). (B) Body compositions of 8-week-old and 12-week-old mice were determined by magnetic resonance imaging using an ECHO MRI (mean ± standard error; n = four mice/group). *, P < 0.05. (A) Data were analyzed by two-way ANOVA; (B) data were analyzed by Student's t test.
FIG. 3.
The absence of collagen VI normalizes the metabolic profile of ob/ob mice and mice receiving a high-fat diet challenge. (A and B) Fasting glucose (left) and circulating glucose levels during an oral glucose tolerance test (right) in 10-week-old (A) and 12-week-old (B) col6KOob/ob and ob/ob mice. (C) Body weight was monitored in collagen VI-null and wild-type littermates. A 12-week high-fat diet challenge was initiated at week 9. In panels A to C, data are represented as means ± standard errors (n = four mice/group). (D) Trichrome Masson stain of subcutaneous skin sections from collagen VI-null and WT mice after a 12-week high-fat diet (HFD) challenge. (E) Circulating triglycerides (Trig) (left panel) and FFA (right panel) were measured during a lipid challenge in 10-week-old col6KOob/ob mice (mean ± SE; n = four mice/group). (F) Fed and fasted triglyceride levels in 10-week-old col6KOob/ob mice. (G) Liver triglyceride content was measured by chloroform-methanol extraction and a colorimetric assay (as described in Materials and Methods) (means ± standard errors; n = four mice/group). (H) H&E-stained pancreatic sections from 10-week-old male mice revealed reduced islet hyperplasia in col6KOob/ob mice compared to ob/ob littermates (upper panel). Sections of the full face of the pancreas were used to quantitate islet area. Islet area was determined at 10× magnification and is expressed as the islet area (μm2)/area of entire pancreas section (μm2) (means ± standard errors; n = five mice/group). Immunofluorescence staining of pancreas sections for insulin (green) and glucagon (red) showed improved α and β cell distributions in col6KOob/ob mice (lower panel). (I) Transmission electron microscopy of epididymal adipose tissue from 16-week-old col6KOob/ob and ob/ob mice. The arrow specifies a representative invaginated caveolae, while the arrowhead specifies an uninvaginated caveolae. The doubleheaded arrow indicates the interstitial space between two adipocytes. (J) Insulin signaling in 10-week-old col6KOob/ob mice and their ob/ob littermates that were injected with 1 U insulin/kg BW and from whom epididymal adipose tissue was collected 0, 5, and 10 min postinjection. The phosphorylation state of AKT was determined by Western blotting directly with specific antibodies for P-AKT and total AKT levels. Band intensities were quantitated with the LI-COR imaging system (LI-COR Biosciences) (means ± standard errors; n = three mice/group). *, P < 0.05; **, P < 0.01 (by Student's t test or by two-way ANOVA).
FIG. 3.
The absence of collagen VI normalizes the metabolic profile of ob/ob mice and mice receiving a high-fat diet challenge. (A and B) Fasting glucose (left) and circulating glucose levels during an oral glucose tolerance test (right) in 10-week-old (A) and 12-week-old (B) col6KOob/ob and ob/ob mice. (C) Body weight was monitored in collagen VI-null and wild-type littermates. A 12-week high-fat diet challenge was initiated at week 9. In panels A to C, data are represented as means ± standard errors (n = four mice/group). (D) Trichrome Masson stain of subcutaneous skin sections from collagen VI-null and WT mice after a 12-week high-fat diet (HFD) challenge. (E) Circulating triglycerides (Trig) (left panel) and FFA (right panel) were measured during a lipid challenge in 10-week-old col6KOob/ob mice (mean ± SE; n = four mice/group). (F) Fed and fasted triglyceride levels in 10-week-old col6KOob/ob mice. (G) Liver triglyceride content was measured by chloroform-methanol extraction and a colorimetric assay (as described in Materials and Methods) (means ± standard errors; n = four mice/group). (H) H&E-stained pancreatic sections from 10-week-old male mice revealed reduced islet hyperplasia in col6KOob/ob mice compared to ob/ob littermates (upper panel). Sections of the full face of the pancreas were used to quantitate islet area. Islet area was determined at 10× magnification and is expressed as the islet area (μm2)/area of entire pancreas section (μm2) (means ± standard errors; n = five mice/group). Immunofluorescence staining of pancreas sections for insulin (green) and glucagon (red) showed improved α and β cell distributions in col6KOob/ob mice (lower panel). (I) Transmission electron microscopy of epididymal adipose tissue from 16-week-old col6KOob/ob and ob/ob mice. The arrow specifies a representative invaginated caveolae, while the arrowhead specifies an uninvaginated caveolae. The doubleheaded arrow indicates the interstitial space between two adipocytes. (J) Insulin signaling in 10-week-old col6KOob/ob mice and their ob/ob littermates that were injected with 1 U insulin/kg BW and from whom epididymal adipose tissue was collected 0, 5, and 10 min postinjection. The phosphorylation state of AKT was determined by Western blotting directly with specific antibodies for P-AKT and total AKT levels. Band intensities were quantitated with the LI-COR imaging system (LI-COR Biosciences) (means ± standard errors; n = three mice/group). *, P < 0.05; **, P < 0.01 (by Student's t test or by two-way ANOVA).
FIG. 4.
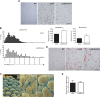
col6KOob/ob mice have larger adipocytes. (A) H&E-stained tissue sections of epididymal adipose tissue from col6KOob/ob mice and their ob/ob littermates. (B) The adipocyte area was calculated from epididymal and mesenteric adipose tissue sections using ImageJ software. The graph on the left shows the percentage of adipocytes in every 100-μm2 area range. Average adipocyte areas in epididymal and mesenteric fat are shown in the right panel. Adipocyte area was determined from four mice/group (>500 cells counted/group). *, P < 0.05 by Student's t test. (C) False-colored scanning electron micrographs of adipocytes from epididymal adipose tissue. (D) Sirius red staining of collagen from epididymal fat. (E) Hydroxyproline content of epididymal fat was measured as an indicator of total collagen content. All experiments (A to E) were performed on 8-week-old mice.
FIG. 5.
col6KOob/ob mice have reduced TGF-β signaling. (A) Expression levels of extracellular matrix genes (TGF-β1, decorin, lumican, elastin, and several MMPs) were measured by qRT-PCR in epididymal fat from 8-week-old col6KOob/ob and ob/ob mice. Expression levels were normalized to 18S rRNA. (B) Lumican expression levels correlate to various metabolic states. Expression levels were determined as described for Fig. 1D and are represented as the fold change. (C) TGF-β1 levels in epididymal adipose tissue from 10-week-old mice treated with 30 mg/kg COOH or vehicle for 14 days. Expression levels were measured by qRT-PCR and were normalized to 18S rRNA. (D) Western blot analysis of TGF-β and the phosphorylation state of TGF-β signaling mediators SMAD2 and SMAD3 in epididymal adipose tissue. Band intensities were quantitated with the LI-COR imaging system (LI-COR Biosciences) and are shown in the lower panel. For panels A to D, data are represented as means ± standard errors (n = four mice/group). *, P < 0.05; **, P < 0.01 (by Student's t test).
FIG. 6.
Absence of collagen VI reduces systemic and adipose tissue-specific inflammation. (A) F4/80 immunohistochemical analysis of epididymal fat tissue sections from col6KOob/ob and ob/ob mice, as an indicator of the degree of macrophage infiltration of the tissue. (B) Quantitative real-time PCR analysis for the inflammatory cytokines F4/80, MCP-1, and SAA3 in epididymal and mesenteric fat. Expression levels of all genes were normalized to 18S rRNA (means ± standard errors; n = four mice/group). (C) qRT-PCR analysis of PPAR-γ expression in mesenteric fat. Expression levels were normalized to 18S rRNA (means ± standard errors; n = four mice/group). (D) Phosphorylation states of ERK, JNK, and p38 MAPK in epididymal adipose tissue from col6KOob/ob and ob/ob mice were determined by Western blotting with specific antibodies for their phosphorylated forms and for total levels. Band intensities were quantitated (means ± standard errors; n = three mice/group). (E) LPS challenge in collagen VI-null and WT mice was performed. Circulating IL-6 and MCP-1 levels were measured (upper panel). mRNA expression levels of inflammatory cytokines IL-6 and tumor necrosis factor alpha (TNFα) was measured in epididymal fat by qRT-PCR and normalized to 18S rRNA 90 min after injection of LPS (lower panel) (means ± standard errors; n = four mice/group). (F) CLS were visualized in F4/80-stained epididymal adipose tissue sections. The single asterisk marks a representative CLS, while the double asterisk indicates a remnant lipid droplet after cell death. (G) Apoptotic adipocytes were visualized by TUNEL staining of epididymal tissue sections of col6KOob/ob and ob/ob mice. Brown staining is indicative of a TUNEL-positive nucleus (arrow), while blue staining (hematoxylin stain) represents a nonreactive nucleus (arrowhead). (H) Endoplasmic reticulum stress was measured by PCR analysis for Xbp-1. Primers spanning the splice junction were used to amplify unspliced and spliced forms of Xbp-1 and were separate on a 3% agarose gel. Unspliced Xbp-1 (Xbp1u) is 171 bp, while the spliced Xbp-1 (Xbp1s) is 145 bp and is a positive indication of endoplasmic reticulum stress. All experiments in this figure were performed with 10-week-old col6KOob/ob and ob/ob mice. *, P < 0.05 (data in panels B, C, D, and F were analyzed by Student's t test; data in panel E were analyzed by two-way ANOVA).
FIG. 7.
Metabolic cage studies of col6KOob/ob mice and their ob/ob littermates. (A) Food intake was measured and is expressed as food intake/gram of body weight (means ± standard errors; n = four mice/group). (B to D) Total locomotion (ambulatory and rearing) (B), oxygen consumption (VO2) (C), and RERs (D) were measured during the course of the day (means ± standard errors; n = four mice/group). All mice used in this experiment were 12 weeks old. *, P < 0.05 by Student's t test or two-way ANOVA.
Similar articles
- Fight against fibrosis in adipose tissue remodeling.
Li S, Gao H, Hasegawa Y, Lu X. Li S, et al. Am J Physiol Endocrinol Metab. 2021 Jul 1;321(1):E169-E175. doi: 10.1152/ajpendo.00558.2020. Epub 2021 May 31. Am J Physiol Endocrinol Metab. 2021. PMID: 34056922 Free PMC article. Review. - Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation.
Spencer M, Yao-Borengasser A, Unal R, Rasouli N, Gurley CM, Zhu B, Peterson CA, Kern PA. Spencer M, et al. Am J Physiol Endocrinol Metab. 2010 Dec;299(6):E1016-27. doi: 10.1152/ajpendo.00329.2010. Epub 2010 Sep 14. Am J Physiol Endocrinol Metab. 2010. PMID: 20841504 Free PMC article. - Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction.
Sun K, Park J, Gupta OT, Holland WL, Auerbach P, Zhang N, Goncalves Marangoni R, Nicoloro SM, Czech MP, Varga J, Ploug T, An Z, Scherer PE. Sun K, et al. Nat Commun. 2014 Mar 19;5:3485. doi: 10.1038/ncomms4485. Nat Commun. 2014. PMID: 24647224 Free PMC article. - Diet-dependent function of the extracellular matrix proteoglycan Lumican in obesity and glucose homeostasis.
Wolff G, Taranko AE, Meln I, Weinmann J, Sijmonsma T, Lerch S, Heide D, Billeter AT, Tews D, Krunic D, Fischer-Posovszky P, Müller-Stich BP, Herzig S, Grimm D, Heikenwälder M, Kao WW, Vegiopoulos A. Wolff G, et al. Mol Metab. 2019 Jan;19:97-106. doi: 10.1016/j.molmet.2018.10.007. Epub 2018 Oct 23. Mol Metab. 2019. PMID: 30409703 Free PMC article. - Architecture and the extracellular matrix: the still unappreciated components of the adipose tissue.
Divoux A, Clément K. Divoux A, et al. Obes Rev. 2011 May;12(5):e494-503. doi: 10.1111/j.1467-789X.2010.00811.x. Epub 2011 Mar 2. Obes Rev. 2011. PMID: 21366833 Review.
Cited by
- Gene expression and protein secretion during human mesenchymal cell differentiation into adipogenic cells.
Amable PR, Teixeira MV, Carias RB, Granjeiro JM, Borojevic R. Amable PR, et al. BMC Cell Biol. 2014 Dec 20;15:46. doi: 10.1186/s12860-014-0046-0. BMC Cell Biol. 2014. PMID: 25526965 Free PMC article. - Endotrophin in the tumor stroma: a new therapeutic target for breast cancer?
Park J, Scherer PE. Park J, et al. Expert Rev Anticancer Ther. 2013 Feb;13(2):111-3. doi: 10.1586/era.12.164. Expert Rev Anticancer Ther. 2013. PMID: 23406549 Free PMC article. Review. No abstract available. - SIRT1 promotes lipid metabolism and mitochondrial biogenesis in adipocytes and coordinates adipogenesis by targeting key enzymatic pathways.
Majeed Y, Halabi N, Madani AY, Engelke R, Bhagwat AM, Abdesselem H, Agha MV, Vakayil M, Courjaret R, Goswami N, Hamidane HB, Elrayess MA, Rafii A, Graumann J, Schmidt F, Mazloum NA. Majeed Y, et al. Sci Rep. 2021 Apr 14;11(1):8177. doi: 10.1038/s41598-021-87759-x. Sci Rep. 2021. PMID: 33854178 Free PMC article. - Fight against fibrosis in adipose tissue remodeling.
Li S, Gao H, Hasegawa Y, Lu X. Li S, et al. Am J Physiol Endocrinol Metab. 2021 Jul 1;321(1):E169-E175. doi: 10.1152/ajpendo.00558.2020. Epub 2021 May 31. Am J Physiol Endocrinol Metab. 2021. PMID: 34056922 Free PMC article. Review. - The role of metformin and resveratrol in the prevention of hypoxia-inducible factor 1α accumulation and fibrosis in hypoxic adipose tissue.
Li X, Li J, Wang L, Li A, Qiu Z, Qi LW, Kou J, Liu K, Liu B, Huang F. Li X, et al. Br J Pharmacol. 2016 Jun;173(12):2001-15. doi: 10.1111/bph.13493. Epub 2016 May 15. Br J Pharmacol. 2016. PMID: 27059094 Free PMC article.
References
- Belvisi, M. G., D. J. Hele, and M. A. Birrell. 2006. Peroxisome proliferator-activated receptor gamma agonists as therapy for chronic airway inflammation. Eur. J. Pharmacol. 533101-109. - PubMed
- Berria, R., L. Wang, D. K. Richardson, J. Finlayson, R. Belfort, T. Pratipanawatr, E. A. De Filippis, S. Kashyap, and L. J. Mandarino. 2006. Increased collagen content in insulin-resistant skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 290E560-E565. - PubMed
- Bidanset, D. J., C. Guidry, L. C. Rosenberg, H. U. Choi, R. Timpl, and M. Hook. 1992. Binding of the proteoglycan decorin to collagen type VI. J. Biol. Chem. 2675250-5256. - PubMed
- Bonaldo, P., P. Braghetta, M. Zanetti, S. Piccolo, D. Volpin, and G. M. Bressan. 1998. Collagen VI deficiency induces early onset myopathy in the mouse: an animal model for Bethlem myopathy. Hum. Mol. Genet. 72135-2140. - PubMed
- Boyd, N. L., H. Park, H. Yi, Y. C. Boo, G. P. Sorescu, M. Sykes, and H. Jo. 2003. Chronic shear induces caveolae formation and alters ERK and Akt responses in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 285H1113-H1122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-DK55758/DK/NIDDK NIH HHS/United States
- T32 DK007513/DK/NIDDK NIH HHS/United States
- T32-DK007513/DK/NIDDK NIH HHS/United States
- R01-CA112023/CA/NCI NIH HHS/United States
- PL1 DK081182/DK/NIDDK NIH HHS/United States
- R01 DK055758/DK/NIDDK NIH HHS/United States
- R01 CA112023/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous