An Xpb mouse model for combined xeroderma pigmentosum and cockayne syndrome reveals progeroid features upon further attenuation of DNA repair - PubMed (original) (raw)
An Xpb mouse model for combined xeroderma pigmentosum and cockayne syndrome reveals progeroid features upon further attenuation of DNA repair
Jaan-Olle Andressoo et al. Mol Cell Biol. 2009 Mar.
Abstract
Patients carrying mutations in the XPB helicase subunit of the basal transcription and nucleotide excision repair (NER) factor TFIIH display the combined cancer and developmental-progeroid disorder xeroderma pigmentosum/Cockayne syndrome (XPCS). Due to the dual transcription repair role of XPB and the absence of animal models, the underlying molecular mechanisms of XPB(XPCS) are largely uncharacterized. Here we show that severe alterations in Xpb cause embryonic lethality and that knock-in mice closely mimicking an XPCS patient-derived XPB mutation recapitulate the UV sensitivity typical for XP but fail to show overt CS features unless the DNA repair capacity is further challenged by crossings to the NER-deficient Xpa background. Interestingly, the Xpb(XPCS) Xpa double mutants display a remarkable interanimal variance, which points to stochastic DNA damage accumulation as an important determinant of clinical diversity in NER syndromes. Furthermore, mice carrying the Xpb(XPCS) mutation together with a point mutation in the second TFIIH helicase Xpd are healthy at birth but display neonatal lethality, indicating that transcription efficiency is sufficient to permit embryonal development even when both TFIIH helicases are crippled. The double-mutant cells exhibit sensitivity to oxidative stress, suggesting a role for endogenous DNA damage in the onset of XPB-associated CS.
Figures
FIG. 1.
Targeting of the mouse gene Xpb. (A) Sequence of the last intron-exon border of the human XPB and XP11BE and the corresponding mouse Xpb alleles. C-to-A transversion (bold) creates a new splice acceptor site (CAG; underlined italics), resulting in a frame-shifted transcript and 40 novel amino acids at the C terminus derived from the frameshift (first five frameshifted amino acids are indicated). A mouse 4-bp insertion in m_Xpb_ creates a similar frameshift (Xpb fs; bold). Due to differences in the nucleotide sequence between mice and humans, in mice the 4-bp insertion leads to substitution of the last 41 amino acids to 85 frame-shifted amino acids (G). (B) Schematic representation of the genomic structure and partial restriction map of the WT and targeted mouse Xpb loci. Black box, exon 15; p(A), polyadenylation signal; †, Xpb fs 4-bp insertion. Probes A, B, and C are indicated with thick black lines. Restriction sites are abbreviated as follows: Nc, NcoI; Bg, BglII; B, BamHI; X, XbaI; C, ClaI. The diagnostic BglII site (Bg) introduced in the sequence is indicated in bold italics. (C) Southern blot analysis of NcoI- and BglII-digested genomic DNA from WT and Xpb fs recombinant ES clones hybridized with probes A, C, and B as indicated. (D) Sequence of the last intron-exon border of mouse WT and Xpb XPCS alleles. The WT splice acceptor site is underlined.Changed nucleotides in the mutated allele (bold) create an additional 5′ splice acceptor site (underlined) and stop codon (TAG; bold) in the altered reading frame only. Amino acids encoded by both WT and Xpb XPCS reading frames are indicated. (E) Genomic structure of the Xpb WT and Xpb XPCS alleles. The asterisk indicates the introduced 5′ splice acceptor site. (F) Southern blot analysis of NcoI-digested genomic DNA from WT ES cells (+/+), Xpb XPCS recombinant ES clones (+/−), and homozygous Xpb XPCS/XPCS mutant mice (−/−) hybridized with probes B and C. (G) Schematic representation of human (NP_000113.1) and mouse (NP_598419.1) XPB proteins derived from alleles depicted in panels A and D. NLS, nuclear localization signal; fs. aa, novel amino acids derived from the frameshift; the C-terminal recognition epitope of 2G12 MAb is indicated with a line. (H) Immunoblot analysis of whole-cell extracts from HeLa cells, two independent homozygous mutant Xpb XPCS MEF lines (−/−), and WT MEFs (+/+). Note that the αXPB MAbs 1B3 and 2G12 recognize conserved epitopes within the N and C termini, respectively, and that the C-terminal epitope is absent in the truncated protein (G). The p62 subunit of TFIIH (stained with MAb 3C9; lower panel) served as qualitative control for loading. (I) Reduction of TFIIH protein levels in homozygous Xpb XPCS primary MEFs visualized by comparative immunofluorescence assay. WT cells were labeled with 0.79-μm latex beads; Xpb XPCS cells were unlabeled (asterisk). Left panel, phase contrast image; right panel, corresponding immunofluorescent image of the p62 subunit of TFIIH. Note the reduced signal in the Xpb XPCS cells. (J) Quantification of the TFIIH level in Xpb XPCS MEFs. The immunofluorescence signal from Xpb XPCS cells was determined (at least 50 nuclei per genotype, two separate experiments with two independent Xpb XPCS and WT cell lines) and expressed as the average percentage of the level in WT cells analyzed on the same microscopic slide. Error bars indicate the SEM between experiments. H.sp., Homo sapiens; M.m., Mus musculus.
FIG. 2.
Cellular DNA repair characteristics. (A) UV-induced (15 J/m2 UVC) unscheduled DNA repair synthesis capacity (UDS) of primary homozygous Xpb XPCS MEFs. A representative experiment (three experiments total) is depicted. The P value indicates the significance of the difference between WT and Xpb XPCS within the representative experiment. Error bars indicate the SEM. (B) Recovery of RNA synthesis after UV (10 J/m2 UVC) irradiation (RRS). A representative experiment (three experiments total) is depicted. The P value indicates the significance of the difference between WT and Xpb XPCS within the representative experiment. Error bars indicate the SEM. (C) UV survival curves averaged from four independent experiments. At least 2 cell lines per genotype were included. Error bars indicate SEM between experiments. (D) Gamma ray survival curves averaged from five independent experiments with two cell lines per genotype. Error bars indicate the SEM between experiments. (E) Reduction of TFIIH protein levels in Xpb XPCS, Xpd TTD single-mutant, and Xpb XPCS Xpd TTD double-mutant primary MEFs by comparative immunofluorescence analysis of p62 subunit of TFIIH. Quantification of the immunofluorescence signal is based on analysis of at least 50 nuclei per genotype in two separate experiments with two independent cell lines per genotype. WT cells are labeled with latex beads, mixed with the mutant cells, and cultured and immunostained on the same microscopic slide. Bars representing cell lines analyzed on the same microscopic slide are depicted side by side. The P value indicates the minimum significant difference between WT versus mutant cell lines analyzed on the same microscopic slide within one experiment. (F) UV survival curves averaged from four independent experiments. At least two cell lines per genotype were included. Error bars indicate the SEM between experiments. (G) Hypersensitivity of the indicated cells to acute oxidative damage. Gamma ray survival curves averaged from two independent experiments with two cell lines per genotype. Error bars indicate the SEM between experiments and lie within the symbol size. (H) Hypersensitivity of Xpb XPCS Xpd TTD and Xpa Xpb XPCS cells to chronic oxidative injury. MEF cells of the indicated genotype were cultured in the continuous presence of the indicated concentration of paraquat for 3 days. Two cell lines per genotype were tested. For reasons of simplicity, on the depicted representative survival experiment, results from two independent cell lines for Xpb XPCS and Xpa Xpb XPCS cells were averaged and error bars depict the SEM between two independent cell lines within the given experiment. For the other genotypes in the given experiment, one cell line per genotype was used and error bars depict the SEM within the experiment.
FIG. 3.
Acute effects of UV-B on the skin of Xpb XPCS mice. H&E staining of dorsal skin sections from shaven mice exposed to daily doses of 500 J/m2 UVB for four consecutive days and sacrificed 1 week after the start of the treatment. Note the moderate epidermal hyperplasia, consisting of an increased number of cell layers (acanthosis, indicated with a bar) in the Xpb XPCS skin and the much more severe effect in Xpa mice as evident from hyperemia (dilated capillaries filled with blood, indicated with a long arrow) and the absence of keratinized and epidermal layers (asterisk), reflecting severe “scaling of the skin.” Note the “swelling” of the nuclei after UV treatment in all genotypes compared to untreated controls (short arrows). Magnification, ×400.
FIG. 4.
Effects of additional NER mutations in Xpb XPCS mice. (A) Kaplan-Meier survival curve of Xpa Xpb XPCS mice. Shown are results for the WT (n = 36), Xpa (n = 34), Xpb XPCS (n = 41), Xpa Xpb XPCS (n = 30), and Xpb XPCS Xpd TTD (n = 5). Please note that for Xpb XPCS Xpd TTD mice, the exact time of death is unknown; it likely occurred within 1 to 2 days after birth (see Table 2 and above). (B) Body weight of Xpa Xpb XPCS mice, plotted as a percentage of age and gender-matched littermate controls. Note the increase in body weight between 4.7 and 17 weeks. Error bars indicate the SEM. (C) Tail suspension test of 7-week-old male mice. The WT mouse depicted displays normal spreading of the hind limbs. Xpa Xpb XPCS mice displayed heterogeneous behavior, ranging from normal to severe cramp-like seizures, spastic movements, and tremors of hind limbs as depicted here. Note also the smaller size of the Xpa Xpb XPCS mouse. (D) Severe kyphosis in a 16-month-old male Xpa Xpb XPCS mouse. (E) Premature testicular tubular atrophy of Xpa Xpb XPCS mice. H&E-stained sections of the testis of 18-month and 7-week-old WT and Xpa Xpb XPCS mice. Note the reduced thickness or absence of the germinal epithelium (bar), reduced occurrence of mature spermatids (arrow), and the increase in interstitial cells (asterisk) in Xpa Xpb XPCS males at 18 months but not at 7 weeks. Magnification, ×100. (F) Photograph of a ∼24-h-old Xpb XPCS Xpd TTD double-mutant mouse and a double-heterozygote littermate. After apparent normal embryogenesis, double-mutant mice failed to grow and died within ∼36 h.
FIG. 5.
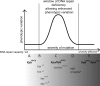
Schematic representation of the relation between the extent of the DNA repair deficiency and the phenotypic variation. With a mild DNA repair defect, limited phenotypic variation is noted, whereas mice with a severe DNA repair defect die too fast to allow stochastic effects to manifest. The biggest phenotypic variation is expected in animals with an intermediate DNA repair defect. Lower panel: the existing mouse models are grouped from A to E based on phenotypic variation and presumed DNA repair (or other) defects. (A) Xpb XPCS animals with a “mild” causative allele exhibit no detectable phenotypic deviation from the WT. (B) In mice mimicking the human “severe” CS, XPCS, or TTD causative mutations, phenotypes are relatively mild, suggesting that the DNA repair capacity is still proficient enough to battle most of the endogenous DNA damage load. (C) Xpa Xpb XPCS mice display the widest phenotypic variation. On top of complete NER deficiency, these animals are also compromised in the removal of non-NER-type oxidative DNA lesions. Some symptoms in Xpa Xpb XPCS mice appeared less penetrant (23% juvenile lethality; kyphosis and/or cachexia in 30% of animals) than others (tubular testicular atrophy was seen in all tested ageing males). Presumably, a random build-up of genome damage in the critical progenitor cells of individual mice may lead to diverse phenotypic consequences both during development and upon aging, depending on the number and tissue context of the affected cells. Xpa Xpd TTD/XPCS mice were also placed within or near group C. Here the effect of the mutation is made “milder” by tissue-specific usage of each Xpd allele product and/or interallelic complementation. (D) Animals with NER progeria (Table 3) die nearly uniformly around weaning, leaving little time for stochastically accumulating lesions to manifest in phenotype. (E) Xpb XPCS Xpd TTD mice have defects in the repair of oxidative DNA damage (Fig. 2G and H) but may, in addition, have defects in activated transcription (18, 35), explaining their failure to survive beyond 1 to 2 days after birth (Table 3). Mouse models generated in this study are indicated (bold).
Similar articles
- Phenotypic heterogeneity in the XPB DNA helicase gene (ERCC3): xeroderma pigmentosum without and with Cockayne syndrome.
Oh KS, Khan SG, Jaspers NG, Raams A, Ueda T, Lehmann A, Friedmann PS, Emmert S, Gratchev A, Lachlan K, Lucassan A, Baker CC, Kraemer KH. Oh KS, et al. Hum Mutat. 2006 Nov;27(11):1092-103. doi: 10.1002/humu.20392. Hum Mutat. 2006. PMID: 16947863 - XPB and XPD helicases in TFIIH orchestrate DNA duplex opening and damage verification to coordinate repair with transcription and cell cycle via CAK kinase.
Fuss JO, Tainer JA. Fuss JO, et al. DNA Repair (Amst). 2011 Jul 15;10(7):697-713. doi: 10.1016/j.dnarep.2011.04.028. Epub 2011 May 14. DNA Repair (Amst). 2011. PMID: 21571596 Free PMC article. Review. - Persistence of repair proteins at unrepaired DNA damage distinguishes diseases with ERCC2 (XPD) mutations: cancer-prone xeroderma pigmentosum vs. non-cancer-prone trichothiodystrophy.
Boyle J, Ueda T, Oh KS, Imoto K, Tamura D, Jagdeo J, Khan SG, Nadem C, Digiovanna JJ, Kraemer KH. Boyle J, et al. Hum Mutat. 2008 Oct;29(10):1194-208. doi: 10.1002/humu.20768. Hum Mutat. 2008. PMID: 18470933 Free PMC article. - XPB: An unconventional SF2 DNA helicase.
Fan L, DuPrez KT. Fan L, et al. Prog Biophys Mol Biol. 2015 Mar;117(2-3):174-181. doi: 10.1016/j.pbiomolbio.2014.12.005. Epub 2015 Jan 30. Prog Biophys Mol Biol. 2015. PMID: 25641424 Review.
Cited by
- Mammalian Resilience Revealed by a Comparison of Human Diseases and Mouse Models Associated With DNA Helicase Deficiencies.
Kohzaki M. Kohzaki M. Front Mol Biosci. 2022 Aug 11;9:934042. doi: 10.3389/fmolb.2022.934042. eCollection 2022. Front Mol Biosci. 2022. PMID: 36032672 Free PMC article. Review. - Cell-autonomous progeroid changes in conditional mouse models for repair endonuclease XPG deficiency.
Barnhoorn S, Uittenboogaard LM, Jaarsma D, Vermeij WP, Tresini M, Weymaere M, Menoni H, Brandt RM, de Waard MC, Botter SM, Sarker AH, Jaspers NG, van der Horst GT, Cooper PK, Hoeijmakers JH, van der Pluijm I. Barnhoorn S, et al. PLoS Genet. 2014 Oct 9;10(10):e1004686. doi: 10.1371/journal.pgen.1004686. eCollection 2014 Oct. PLoS Genet. 2014. PMID: 25299392 Free PMC article. - Genetic correction of stem cells in the treatment of inherited diseases and focus on xeroderma pigmentosum.
Rouanet S, Warrick E, Gache Y, Scarzello S, Avril MF, Bernerd F, Magnaldo T. Rouanet S, et al. Int J Mol Sci. 2013 Oct 9;14(10):20019-36. doi: 10.3390/ijms141020019. Int J Mol Sci. 2013. PMID: 24113582 Free PMC article. Review. - Age-related motor neuron degeneration in DNA repair-deficient Ercc1 mice.
de Waard MC, van der Pluijm I, Zuiderveen Borgesius N, Comley LH, Haasdijk ED, Rijksen Y, Ridwan Y, Zondag G, Hoeijmakers JH, Elgersma Y, Gillingwater TH, Jaarsma D. de Waard MC, et al. Acta Neuropathol. 2010 Oct;120(4):461-75. doi: 10.1007/s00401-010-0715-9. Epub 2010 Jul 4. Acta Neuropathol. 2010. PMID: 20602234 Free PMC article. - DNA damage responses in ageing.
da Silva PFL, Schumacher B. da Silva PFL, et al. Open Biol. 2019 Nov 29;9(11):190168. doi: 10.1098/rsob.190168. Epub 2019 Nov 20. Open Biol. 2019. PMID: 31744423 Free PMC article. Review.
References
- Andressoo, J. O., and J. H. Hoeijmakers. 2005. Transcription-coupled repair and premature ageing. Mutat. Res. 577179-194. - PubMed
- Andressoo, J. O., J. Jans, J. de Wit, F. Coin, D. Hoogstraten, M. van de Ven, W. Toussaint, J. Huijmans, H. B. Thio, W. J. van Leeuwen, J. de Boer, J. M. Egly, J. H. Hoeijmakers, G. T. van der Horst, and J. R. Mitchell. 2006. Rescue of progeria in trichothiodystrophy by homozygous lethal Xpd alleles. PLoS Biol. 4e322. - PMC - PubMed
- Andressoo, J. O., J. R. Mitchell, J. de Wit, D. Hoogstraten, M. Volker, W. Toussaint, E. Speksnijder, R. B. Beems, H. van Steeg, J. Jans, C. I. de Zeeuw, N. G. Jaspers, A. Raams, A. R. Lehmann, W. Vermeulen, J. H. Hoeijmakers, and G. T. van der Horst. 2006. An Xpd mouse model for the combined xeroderma pigmentosum/Cockayne syndrome exhibiting both cancer predisposition and segmental progeria. Cancer Cell 10121-132. - PubMed
- Bahar, R., C. H. Hartmann, K. A. Rodriguez, A. D. Denny, R. A. Busuttil, M. E. Dolle, R. B. Calder, G. B. Chisholm, B. H. Pollock, C. A. Klein, and J. Vijg. 2006. Increased cell-to-cell variation in gene expression in ageing mouse heart. Nature 4411011-1014. - PubMed
- Bartke, A. 2005. Minireview: role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology 1463718-3723. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 ES011044/ES/NIEHS NIH HHS/United States
- 1P01AG17242-02/AG/NIA NIH HHS/United States
- 233424/ERC_/European Research Council/International
- P01 AG017242/AG/NIA NIH HHS/United States
- 1U01 ES011044/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials