Direct link between RACK1 function and localization at the ribosome in vivo - PubMed (original) (raw)
Direct link between RACK1 function and localization at the ribosome in vivo
Scott M Coyle et al. Mol Cell Biol. 2009 Mar.
Abstract
The receptor for activated C-kinase (RACK1), a conserved protein implicated in numerous signaling pathways, is a stoichiometric component of eukaryotic ribosomes located on the head of the 40S ribosomal subunit. To test the hypothesis that ribosome association is central to the function of RACK1 in vivo, we determined the 2.1-A crystal structure of RACK1 from Saccharomyces cerevisiae (Asc1p) and used it to design eight mutant versions of RACK1 to assess roles in ribosome binding and in vivo function. Conserved charged amino acids on one side of the beta-propeller structure were found to confer most of the 40S subunit binding affinity, whereas an adjacent conserved and structured loop had little effect on RACK1-ribosome association. Yeast mutations that confer moderate to strong defects in ribosome binding mimic some phenotypes of a RACK1 deletion strain, including increased sensitivity to drugs affecting cell wall biosynthesis and translation elongation. Furthermore, disruption of RACK1's position at the 40S ribosomal subunit results in the failure of the mRNA binding protein Scp160 to associate with actively translating ribosomes. These results provide the first direct evidence that RACK1 functions from the ribosome, implying a physical link between the eukaryotic ribosome and cell signaling pathways in vivo.
Figures
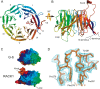
FIG. 1.
Crystal structure of RACK1. (A) Cartoon representation of the RACK1 crystal structure viewed from the top, the ribosome-binding face, to show the overall seven-bladed β-propeller architecture. The individual blades have been labeled I to VII and are colored using the CHAINBOWs scheme of MacPymol. (B) Cartoon representation of the RACK1 crystal structure viewed from the side. From this view, the two dramatic insertions are visible, labeled knob and loop, respectively. The twisting, extended interaction between the N (blue) and C (red) termini is also visible from this view. (C) Space-filling views of the seven-bladed β-propeller architecture of the yeast Gβ and our RACK1 crystal structure, showing the unusual asymmetry of the RACK1 structure. (D) Stick diagram of the structured knob region of the RACK1 structure with the corresponding 2Fobs-Fcalc electron density map shown contoured at 1.0 sigma. All ray-traced images were generated using MacPymol (13). Surface electrostatics were also calculated by using MacPymol. The conservation heat plot of the RACK1 surface was generated by using ConSurf (3) using a multiple sequence alignment we generated (unpublished data) using MUSCLE (14).
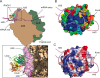
FIG. 2.
The RACK1-40S interface. (A) Cartoon schematic showing the positioning of RACK1 on the 40S ribosomal subunit as aligned using a least-squares superimposition function in Coot (15). The model positions RACK1 near the mRNA exit tunnel in close proximity to helices 39 and 40 of the 18S rRNA. A close-up view of this interaction with cryo-EM density for the 40S ribosome modeled shows that the top portion of RACK1, including the structured knob, faces the ribosome, while the bottom and sides of the protein are solvent facing and generally accessible. (B) Space-filling view of RACK1, colored according to electrostatic surface potential, with the positions of helices 39 and 40 modeled. Blue, positive charge; red, negative charge. (C) Space-filling view of RACK1, colored according to sequence conservation with the positions of helices 39 and 40 modeled. A gradient of blue to red indicates the degree of phylogenetic conservation, with dark blue indicating high conservation and red indicating low conservation.
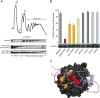
FIG. 3.
Effects of RACK1 mutations on ribosome binding in vivo. (A) Representative data from polysome Western blot assays performed to determine the extent of ribosome association in vivo. A model polysome trace is shown, with the mRNP, 40S, 60S, 80S, and polysome fractions indicated. Western blots against RACK1 for fractions collected across the gradient from the wild-type, R38D K40E, and K87A R90A mutants are shown; fractions corresponding to free and ribosome-associated RACK1 are indicated. (B) Bar graph displaying the extent of ribosome association in each mutant tested, with error bars extending ± 2 standard deviations. Bars were colored to highlight the extent of the binding defect and for reference in the color-coding of the RACK1 surface in panel C. A Western blot showing the total RACK1 protein levels for each mutant is shown beneath each corresponding bar. (C) Space-filling view of RACK1 colored according to severity of binding defect in accordance with the findings shown in panel B, with the positions of helices 39 and 40 modeled. Residues corresponding to the (65-67)Δ and (Loop)Δ mutants are not colored.
FIG. 4.
RACK1 mutant phenotyping. Summary of phenotypes observed for the RACK1 mutant, RACK1Δ, and wild-type yeast strains under a variety of environmental conditions. For YPD, SCD-Ura, potassium disulfite, and rapamycin, the relative growth rates are indicated. +++, normal growth; ++, slow growth; +, very slow growth; −, no growth. For calcofluor white, a B indicates that cells were blue, while a W indicates that cells were white. For invasive growth assays, a Y indicates that cells were competent for invasive growth, while an N indicates that the cells were not. The percentage of RACK1 associated with ribosomes in each mutant is included for reference. Representative images of the calcofluor white staining assay are included for each mutant to illustrate the blue and white phenotypes.
FIG. 5.
Effect of RACK1's ribosome localization on Scp160 association with polysomes. Upper panel, cartoon of the hypothesized RACK1/Scp160 scaffold at the ribosome; direct interaction could deliver specific mRNAs to the ribosome. Lower panel, polysome Western blot assay for Scp160 present in samples from wild-type and RACK1 R38D K40E mutant yeast strains.
Similar articles
- Communication between RACK1/Asc1 and uS3 (Rps3) is essential for RACK1/Asc1 function in yeast Saccharomyces cerevisiae.
Singh N, Jindal S, Ghosh A, Komar AA. Singh N, et al. Gene. 2019 Jul 20;706:69-76. doi: 10.1016/j.gene.2019.04.087. Epub 2019 May 1. Gene. 2019. PMID: 31054365 Free PMC article. - Asc1p/RACK1 Connects Ribosomes to Eukaryotic Phosphosignaling.
Schmitt K, Smolinski N, Neumann P, Schmaul S, Hofer-Pretz V, Braus GH, Valerius O. Schmitt K, et al. Mol Cell Biol. 2017 Jan 19;37(3):e00279-16. doi: 10.1128/MCB.00279-16. Print 2017 Feb 1. Mol Cell Biol. 2017. PMID: 27821475 Free PMC article. - Capturing the Asc1p/_R_eceptor for _A_ctivated _C K_inase 1 (RACK1) Microenvironment at the Head Region of the 40S Ribosome with Quantitative BioID in Yeast.
Opitz N, Schmitt K, Hofer-Pretz V, Neumann B, Krebber H, Braus GH, Valerius O. Opitz N, et al. Mol Cell Proteomics. 2017 Dec;16(12):2199-2218. doi: 10.1074/mcp.M116.066654. Epub 2017 Oct 5. Mol Cell Proteomics. 2017. PMID: 28982715 Free PMC article. - Regulation of eukaryotic translation by the RACK1 protein: a platform for signalling molecules on the ribosome.
Nilsson J, Sengupta J, Frank J, Nissen P. Nilsson J, et al. EMBO Rep. 2004 Dec;5(12):1137-41. doi: 10.1038/sj.embor.7400291. EMBO Rep. 2004. PMID: 15577927 Free PMC article. Review. - Structural analysis of ribosomal RACK1 and its role in translational control.
Nielsen MH, Flygaard RK, Jenner LB. Nielsen MH, et al. Cell Signal. 2017 Jul;35:272-281. doi: 10.1016/j.cellsig.2017.01.026. Epub 2017 Feb 2. Cell Signal. 2017. PMID: 28161490 Review.
Cited by
- The deoxyhypusine synthase mutant dys1-1 reveals the association of eIF5A and Asc1 with cell wall integrity.
Galvão FC, Rossi D, Silveira Wda S, Valentini SR, Zanelli CF. Galvão FC, et al. PLoS One. 2013;8(4):e60140. doi: 10.1371/journal.pone.0060140. Epub 2013 Apr 1. PLoS One. 2013. PMID: 23573236 Free PMC article. - Cell Signaling and Stress Responses.
Hotamisligil GS, Davis RJ. Hotamisligil GS, et al. Cold Spring Harb Perspect Biol. 2016 Oct 3;8(10):a006072. doi: 10.1101/cshperspect.a006072. Cold Spring Harb Perspect Biol. 2016. PMID: 27698029 Free PMC article. Review. - Ribosomal RACK1:Protein Kinase C βII Phosphorylates Eukaryotic Initiation Factor 4G1 at S1093 To Modulate Cap-Dependent and -Independent Translation Initiation.
Dobrikov MI, Dobrikova EY, Gromeier M. Dobrikov MI, et al. Mol Cell Biol. 2018 Sep 14;38(19):e00304-18. doi: 10.1128/MCB.00304-18. Print 2018 Oct 1. Mol Cell Biol. 2018. PMID: 30012863 Free PMC article. - Interactions between the mRNA and Rps3/uS3 at the entry tunnel of the ribosomal small subunit are important for no-go decay.
Simms CL, Kim KQ, Yan LL, Qiu J, Zaher HS. Simms CL, et al. PLoS Genet. 2018 Nov 26;14(11):e1007818. doi: 10.1371/journal.pgen.1007818. eCollection 2018 Nov. PLoS Genet. 2018. PMID: 30475795 Free PMC article. - Trypanosomatid RACK1 orthologs show functional differences associated with translation despite similar roles in Leishmania pathogenesis.
Choudhury K, Cardenas D, Pullikuth AK, Catling AD, Aiyar A, Kelly BL. Choudhury K, et al. PLoS One. 2011;6(6):e20710. doi: 10.1371/journal.pone.0020710. Epub 2011 Jun 3. PLoS One. 2011. PMID: 21677780 Free PMC article.
References
- Abramoff, M. D., P. J. Magelhaes, and S. J. Ram. 2004. Image processing with ImageJ. Biophotonics Int. 1136-42.
- Adams, P. D., R. W. Grosse-Kunstleve, L. W. Hung, T. R. Ioerger, A. J. McCoy, N. W. Moriarty, R. J. Read, J. C. Sacchettini, N. K. Sauter, and T. C. Terwilliger. 2002. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D 581948-1954. - PubMed
- Armon, A., D. Graur, and N. Ben-Tal. 2001. ConSurf: an algorithmic tool for the identification of functional regions in proteins by surface mapping of phylogenetic information. J. Mol. Biol. 307447-463. - PubMed
- Basmaji, F., H. Martin-Yken, F. Durand, A. Dagkessamanskaia, C. Pichereaux, M. Rossignol, and J. Francois. 2006. The ‘interactome’ of the Knr4/Smi1, a protein implicated in coordinating cell wall synthesis with bud emergence in Saccharomyces cerevisiae. Mol. Genet. Genomics 275217-230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases