Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes - PubMed (original) (raw)
Randomized Controlled Trial
doi: 10.2337/dc08-1863. Epub 2008 Dec 29.
Affiliations
- PMID: 19114612
- PMCID: PMC2660449
- DOI: 10.2337/dc08-1863
Randomized Controlled Trial
Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes
James F List et al. Diabetes Care. 2009 Apr.
Abstract
Objective: Dapagliflozin, a novel inhibitor of renal sodium-glucose cotransporter 2, allows an insulin-independent approach to improve type 2 diabetes hyperglycemia. In this multiple-dose study we evaluated the safety and efficacy of dapagliflozin in type 2 diabetic patients.
Research design and methods: Type 2 diabetic patients were randomly assigned to one of five dapagliflozin doses, metformin XR, or placebo for 12 weeks. The primary objective was to compare mean change from baseline in A1C. Other objectives included comparison of changes in fasting plasma glucose (FPG), weight, adverse events, and laboratory measurements.
Results: After 12 weeks, dapagliflozin induced moderate glucosuria (52-85 g urinary glucose/day) and demonstrated significant glycemic improvements versus placebo (DeltaA1C -0.55 to -0.90% and DeltaFPG -16 to -31 mg/dl). Weight loss change versus placebo was -1.3 to -2.0 kg. There was no change in renal function. Serum uric acid decreased, serum magnesium increased, serum phosphate increased at higher doses, and dose-related 24-h urine volume and hematocrit increased, all of small magnitude. Treatment-emergent adverse events were similar across all groups.
Conclusions: Dapagliflozin improved hyperglycemia and facilitates weight loss in type 2 diabetic patients by inducing controlled glucosuria with urinary loss of approximately 200-300 kcal/day. Dapagliflozin treatment demonstrated no persistent, clinically significant osmolarity, volume, or renal status changes.
Trial registration: ClinicalTrials.gov NCT00263276.
Figures
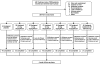
Figure 1
Patient disposition and study design. T2DM, type 2 diabetic.
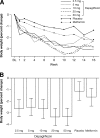
Figure 2
Changes in glycemic parameters. A: Adjusted mean change from baseline in A1C at week 12 (LOCF). B: Mean change in FPG over time (observed values). C: Adjusted mean change from baseline in postprandial glucose area under the curve (AUC) during a 75-g oral glucose tolerance test at week 12 (LOCF). D: Change from baseline in 24-h urinary glucose (grams) normalized for urinary creatinine (grams) at week 12 (LOCF). Displayed are means and 95% CIs (A, B, and D). When statistical significance was achieved with dapagliflozin groups compared with placebo, Dunnett's multiplicity adjustment was used. P values versus placebo at week 12.
Figure 3
Percent changes in weight. A: Percent change from baseline in weight over the 12-week treatment period and 4-week follow-up period (observed values). B: Adjusted mean percent change from baseline in weight after 12 weeks of treatment (LOCF). Displayed are means and 95% CIs.
Similar articles
- Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial.
Wilding JP, Woo V, Soler NG, Pahor A, Sugg J, Rohwedder K, Parikh S; Dapagliflozin 006 Study Group. Wilding JP, et al. Ann Intern Med. 2012 Mar 20;156(6):405-15. doi: 10.7326/0003-4819-156-6-201203200-00003. Ann Intern Med. 2012. PMID: 22431673 Clinical Trial. - Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years.
Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S; Dapagliflozin 006 Study Group. Wilding JP, et al. Diabetes Obes Metab. 2014 Feb;16(2):124-36. doi: 10.1111/dom.12187. Epub 2013 Aug 29. Diabetes Obes Metab. 2014. PMID: 23911013 Clinical Trial. - Exploring the potential of the SGLT2 inhibitor dapagliflozin in type 1 diabetes: a randomized, double-blind, placebo-controlled pilot study.
Henry RR, Rosenstock J, Edelman S, Mudaliar S, Chalamandaris AG, Kasichayanula S, Bogle A, Iqbal N, List J, Griffen SC. Henry RR, et al. Diabetes Care. 2015 Mar;38(3):412-9. doi: 10.2337/dc13-2955. Epub 2014 Sep 30. Diabetes Care. 2015. PMID: 25271207 Clinical Trial. - [Dapagliflozin, the first SGLT-2 inhibitor in the treatment of type 2 diabetes].
Albarrán OG, Ampudia-Blasco FJ. Albarrán OG, et al. Med Clin (Barc). 2013 Sep;141 Suppl 2:36-43. doi: 10.1016/S0025-7753(13)70062-9. Med Clin (Barc). 2013. PMID: 24444523 Review. Spanish. - Dapagliflozin: a review of its use in type 2 diabetes mellitus.
Plosker GL. Plosker GL. Drugs. 2012 Dec 3;72(17):2289-312. doi: 10.2165/11209910-000000000-00000. Drugs. 2012. PMID: 23170914 Review.
Cited by
- Renal hyperfiltration related to diabetes mellitus and obesity in human disease.
Sasson AN, Cherney DZ. Sasson AN, et al. World J Diabetes. 2012 Jan 15;3(1):1-6. doi: 10.4239/wjd.v3.i1.1. World J Diabetes. 2012. PMID: 22253940 Free PMC article. - The Use of SGLT-2 Inhibitors in Type 2 Diabetes and Heart Failure.
Riggs K, Ali H, Taegtmeyer H, Gutierrez AD. Riggs K, et al. Metab Syndr Relat Disord. 2015 Sep;13(7):292-7. doi: 10.1089/met.2015.0038. Epub 2015 Jun 30. Metab Syndr Relat Disord. 2015. PMID: 26125313 Free PMC article. Review. - SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects.
Ferrannini E, Solini A. Ferrannini E, et al. Nat Rev Endocrinol. 2012 Feb 7;8(8):495-502. doi: 10.1038/nrendo.2011.243. Nat Rev Endocrinol. 2012. PMID: 22310849 Review. - Why Do SGLT2 inhibitors inhibit only 30-50% of renal glucose reabsorption in humans?
Liu JJ, Lee T, DeFronzo RA. Liu JJ, et al. Diabetes. 2012 Sep;61(9):2199-204. doi: 10.2337/db12-0052. Diabetes. 2012. PMID: 22923645 Free PMC article. - Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy.
Rosenstock J, Vico M, Wei L, Salsali A, List JF. Rosenstock J, et al. Diabetes Care. 2012 Jul;35(7):1473-8. doi: 10.2337/dc11-1693. Epub 2012 Mar 23. Diabetes Care. 2012. PMID: 22446170 Free PMC article. Clinical Trial.
References
- Kles KA, Vinik AI: Pathophysiology and treatment of diabetic peripheral neuropathy: the case for diabetic neurovascular function as an essential component. Curr Diabetes Rev 2: 131– 145, 2006 - PubMed
- Nazimek-Siewniak B, Moczulski D, Grzeszczak W: Risk of macrovascular and microvascular complications in type 2 diabetes: results of longitudinal study design. J Diabetes Complications 16: 271– 276, 2002 - PubMed
- Rahman S, Rahman T, Ismail AA, Rashid AR: Diabetes-associated macrovasculopathy: pathophysiology and pathogenesis. Diabetes Obes Metab 9: 767– 780, 2007 - PubMed
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW: 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359: 1577– 1589, 2008 - PubMed
- Andrews WJ, Vasquez B, Nagulesparan M, Klimes I, Foley J, Unger R, Reaven GM: Insulin therapy in obese, non-insulin-dependent diabetes induces improvements in insulin action and secretion that are maintained for two weeks after insulin withdrawal. Diabetes 33: 634– 642, 1984 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical