The yeast Sup35NM domain propagates as a prion in mammalian cells - PubMed (original) (raw)
The yeast Sup35NM domain propagates as a prion in mammalian cells
Carmen Krammer et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Prions are infectious, self-propagating amyloid-like protein aggregates of mammals and fungi. We have studied aggregation propensities of a yeast prion domain in cell culture to gain insights into general mechanisms of prion replication in mammalian cells. Here, we report the artificial transmission of a yeast prion across a phylogenetic kingdom. HA epitope-tagged yeast Sup35p prion domain NM was stably expressed in murine neuroblastoma cells. Although cytosolically expressed NM-HA remained soluble, addition of fibrils of bacterially produced Sup35NM to the medium efficiently induced appearance of phenotypically and biochemically distinct NM-HA aggregates that were inherited by daughter cells. Importantly, NM-HA aggregates also were infectious to recipient mammalian cells expressing soluble NM-HA and, to a lesser extent, to yeast. The fact that the yeast Sup35NM domain can propagate as a prion in neuroblastoma cells strongly argues that cellular mechanisms support prion-like inheritance in the mammalian cytosol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
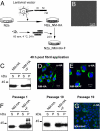
Induction of heritable NM-HA aggregates by exogenous NM fibrils. (A) Schematic diagram of the experiment. A bulk population of N2a cells stably expressing HA-tagged Sup35p yeast prion domain NM was generated by lentiviral transduction (N2a_NM-HA). Cells were exposed to recombinant NM fibrils and subsequently passaged (N2a_NM-HA+F). (B) Atomic force microscopy analysis of fibrillized recombinant NM (10 μM in PBS). (Scale bar: 1 μm.) (C) Sedimentation assay of NM-HA in control cells and 48 h after aggregate induction by exogenous recombinant NM fibrils (NM-HA+F). S indicates supernatant; P, pellet. (D and E) Confocal microscopy analysis of NM-HA with (D) and without (E) exposure to recombinant NM fibrils. (Scale bar: 10 μm.) (F) N2a_NM-HA cells were exposed to NM fibril preparations and subsequently passaged 1 to 10 times. Shown are sedimentation assays of untreated (N2a_NM-HA) or fibril-treated (N2a_NM-HA+F) cells. (G) Confocal microscopy analysis 10 passages after cell exposure to fibrils. (Scale bar: 10 μm.) Antibody (C–G): anti-HA.
Fig. 2.
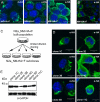
Distinct aggregate phenotypes in progeny cells. (A and B) Higher-resolution images of N2a_NM-HA+F cells displaying distinct aggregate phenotypes. (C) Schematic diagram of the isolation of N2a_NM-HA subclones with distinct aggregate phenotypes. (D) Higher-resolution images of subclones demonstrate inheritance of distinct aggregate phenotypes by progeny cells. Note that clones 4G and 11C displayed no visible NM-HA aggregates. Antibody: anti-HA. (Scale bars: 10 μm.) (E) Relative expression levels of NM-HA by individual clones as detected by Western blot analysis. Antibody: anti-HA. GAPDH detection serves as a loading control.
Fig. 3.
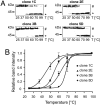
NM-HA aggregates from individual clones exhibit distinct biochemical properties. (A) Relative resistance of NM-HA aggregates to thermal denaturation in the presence of SDS from individual cell clones using SDS/PAGE. Cell lysates were mixed with 1% SDS, and aliquots were incubated at different temperatures. To ensure comparable NM-HA levels, different amounts of lysates were loaded for individual clones. (B) Band intensities of SDS-soluble proteins were plotted against temperature and fitted to a sigmoidal function. Statistical analysis was performed by using the 2-way ANOVA with Bonferroni's multiple-comparison test (P < 0.0001).
Fig. 4.
NM-HA aggregates are infectious. (A) Schematic diagram of the experiment. The bulk population of N2a_NM-HA cells was exposed to cell lysates of N2a_NM-HA+F subclones 1C and 5D displaying NM-HA aggregates or to cell lysates of bulk N2a_NM-HA cells expressing soluble NM-HA. (B) Confocal microscopy analysis of wild-type N2a (control) and N2a_NM-HA bulk cells exposed to cell extracts 1 week after exposure. Individual cells within the population are shown. Note that the aggregate phenotype was not precisely phenocopied upon induction in the recipient cells. Antibody: anti-HA. (Scale bar: 10 μm.)
Fig. 5.
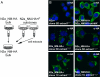
Clonal influence on NM-HA aggregate phenotype. (A) Schematic diagram of the experiment. Individual clones derived from the bulk population of N2a_NM-HA cells were exposed to recombinant NM fibrils. (B) The majority of cells in a given clonal population exhibit a distinct aggregate phenotype as assessed by confocal microscopy using anti-HA antibodies. (Scale bar: 10 μm.) (C) Relative NM-HA expression levels in N2a_NM-HA cell clones before exposure to recombinant NM fibrils. The blot was probed with anti-HA antibodies, and GAPDH was used to demonstrate comparable protein loading.
Fig. 6.
NM-HA amyloids extracted from N2a cells are infectious to yeast. Cell extracts from N2a_NM-HA clones exhibiting visible aggregates (1C, 2E, 3B, 5D), and bulk N2a_NM-HA cells as a negative control were used to induce [PSI+] in the yeast strain 74-D694. A total of 2,000 transformants per inoculum were tested. Recovered colonies were streaked on 1/2 YPD plates to identify possible [PSI+] isolates by color. Such colonies were found only after transformation of cell extracts from clones 1C and 5D (0.2% or 0.05% of ≈2,000 total transformants tested, respectively). Plates with 6 transformants of clone 1C, clone 5D, or bulk cells are shown. [_psi_−] indicates negative control; [PSI+], positive control.
Similar articles
- Prion protein/protein interactions: fusion with yeast Sup35p-NM modulates cytosolic PrP aggregation in mammalian cells.
Krammer C, Suhre MH, Kremmer E, Diemer C, Hess S, Schätzl HM, Scheibel T, Vorberg I. Krammer C, et al. FASEB J. 2008 Mar;22(3):762-73. doi: 10.1096/fj.07-8733com. Epub 2007 Oct 10. FASEB J. 2008. PMID: 17928365 - Horizontal Transmission of Cytosolic Sup35 Prions by Extracellular Vesicles.
Liu S, Hossinger A, Hofmann JP, Denner P, Vorberg IM. Liu S, et al. mBio. 2016 Jul 12;7(4):e00915-16. doi: 10.1128/mBio.00915-16. mBio. 2016. PMID: 27406566 Free PMC article. - Sup35p in Its Soluble and Prion States Is Packaged inside Extracellular Vesicles.
Kabani M, Melki R. Kabani M, et al. mBio. 2015 Aug 18;6(4):e01017-15. doi: 10.1128/mBio.01017-15. mBio. 2015. PMID: 26286691 Free PMC article. - Life cycle of cytosolic prions.
Hofmann J, Vorberg I. Hofmann J, et al. Prion. 2013 Sep-Oct;7(5):369-77. doi: 10.4161/pri.26414. Epub 2013 Sep 10. Prion. 2013. PMID: 24021964 Free PMC article. Review. - Amyloid formation of a yeast prion determinant.
Scheibel T. Scheibel T. J Mol Neurosci. 2004;23(1-2):13-22. doi: 10.1385/JMN:23:1-2:013. J Mol Neurosci. 2004. PMID: 15126688 Review.
Cited by
- Yeast prions: structure, biology, and prion-handling systems.
Wickner RB, Shewmaker FP, Bateman DA, Edskes HK, Gorkovskiy A, Dayani Y, Bezsonov EE. Wickner RB, et al. Microbiol Mol Biol Rev. 2015 Mar;79(1):1-17. doi: 10.1128/MMBR.00041-14. Microbiol Mol Biol Rev. 2015. PMID: 25631286 Free PMC article. Review. - Vaccines for prion diseases: a realistic goal?
Napper S, Schatzl HM. Napper S, et al. Cell Tissue Res. 2023 Apr;392(1):367-392. doi: 10.1007/s00441-023-03749-7. Epub 2023 Feb 11. Cell Tissue Res. 2023. PMID: 36764940 Free PMC article. Review. - Membrane composition and lipid to protein ratio modulate amyloid kinetics of yeast prion protein.
Bandyopadhyay A, Sannigrahi A, Chattopadhyay K. Bandyopadhyay A, et al. RSC Chem Biol. 2021 Feb 5;2(2):592-605. doi: 10.1039/d0cb00203h. eCollection 2021 Apr 1. RSC Chem Biol. 2021. PMID: 34458802 Free PMC article. - Extracellular Vesicle Separation Techniques Impact Results from Human Blood Samples: Considerations for Diagnostic Applications.
Tzaridis T, Bachurski D, Liu S, Surmann K, Babatz F, Gesell Salazar M, Völker U, Hallek M, Herrlinger U, Vorberg I, Coch C, Reiners KS, Hartmann G. Tzaridis T, et al. Int J Mol Sci. 2021 Aug 26;22(17):9211. doi: 10.3390/ijms22179211. Int J Mol Sci. 2021. PMID: 34502122 Free PMC article. - Cellular aspects of prion replication in vitro.
Grassmann A, Wolf H, Hofmann J, Graham J, Vorberg I. Grassmann A, et al. Viruses. 2013 Jan 22;5(1):374-405. doi: 10.3390/v5010374. Viruses. 2013. PMID: 23340381 Free PMC article. Review.
References
- Telling GC, et al. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science. 1996;274:2079–2082. - PubMed
- Cox BS. [PSI], a cytoplasmic suppressor of super-suppressors in yeast. Heredity. 1965;20:505–521.
- Wickner RB. [URE3] as an altered URE2 protein: evidence for a prion analog in Saccharomyces cerevisiae. Science. 1994;264:566–569. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases