Effect of interleukin-1beta on osteogenic protein 1-induced signaling in adult human articular chondrocytes - PubMed (original) (raw)
Effect of interleukin-1beta on osteogenic protein 1-induced signaling in adult human articular chondrocytes
Amel M Elshaier et al. Arthritis Rheum. 2009 Jan.
Abstract
Objective: Two major receptor-activated Smad (R-Smad) signaling pathways, bone morphogenetic protein (BMP) and MAPK, were examined in a model of interleukin-1beta (IL-1beta)-induced cartilage degeneration to investigate the effect of IL-1beta on osteogenic protein 1 (OP-1) signaling in adult human articular chondrocytes.
Methods: Chondrocytes from the ankles of 26 normal human donors were cultured in high-density monolayers in serum-free medium. The effect of IL-1beta on BMP receptors was studied by reverse transcription-polymerase chain reaction and flow cytometry. Phosphorylation of R-Smads was tested in cells treated with IL-1beta (10 ng/ml), OP-1 (100 ng/ml), or the combination of IL-1beta and OP-1. Cell lysates were analyzed by Western blotting with polyclonal antibodies against 2 R-Smad phosphorylation sites (BMP- and MAPK-mediated) or with total, nonphosphorylated R-Smad as a control. To identify which MAPKs play a role in IL-1beta activation of the linker region, chondrocytes were preincubated with specific MAPK inhibitors (PD98059 for MAP/ERK, SP600125 for JNK, and SB203580 for p38).
Results: IL-1beta reduced the number of activin receptor-like kinase 2 (ALK-2) and ALK-3 receptors, inhibited expression of Smad1 and Smad6, delayed and prematurely terminated the onset of OP-1-mediated R-Smad phosphorylation, and affected nuclear translocation of R-Smad/Smad4 complexes. The alternative phosphorylation of R-Smad in the linker region via the MAPK pathway (primarily p38 and JNK) was observed to be a possible mechanism through which IL-1beta offsets OP-1 signaling and the response to OP-1. Conversely, OP-1 was found to directly inhibit phosphorylation of p38.
Conclusion: These findings describe new mechanisms of the crosstalk between OP-1 and IL-1beta in chondrocytes. The study also identifies potential targets for therapeutic interventions in the treatment of cartilage-degenerative processes.
Figures
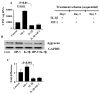
Figure 1. Effect of IL-1β, OP-1 or combined IL-1β+OP-1 on PG synthesis (Fig. A) and aggrecan gene expression (Fig. B-C)
A, The data are presented as CPM normalized to the cellular DNA content. The histograms represent the mean ± SD of four independent experiments; OP-1 vs mini-ITS, P<0.01; IL-1β vs mini-ITS, P<0.01. B, representative PCR gel for aggrecan expression. C, corresponding histograms represent the mean ± SD of the relative density of the bands after densitometric scanning of 1% agarose gel from four independent experiments. The relative densities of the bands were normalized to that of GAPDH to control sample variations. OP-1 vs mini-ITS, P<0.001; IL-1β vs mini-ITS, P<0.001.
Figure 2. Gene expression for endogenous OP-1 and its downstream signaling mediators
A, PCR gels with corresponding size of the band. B. Summary of the effect of IL-1β on the gene expression of OP-1 and its downstream signaling mediators.  Expression was up-regulated;
Expression was up-regulated; expression was down-regulated;
expression was down-regulated; expression did not change.
expression did not change.
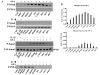
Figure 3. Changes in the number of BMP receptors after IL-1β stimulation
Flow cytometric analysis of ALK-2, 3, 6 and BMPR-II receptors with IL-1β treatment. A, B & C, histograms represent changes in ALK-3 (BMPR-IA) receptor in chondrocytes treated with IL-1β for 24, 48 and 72 hours respectively. D, Histogram represents changes in ALK-2 receptor in chondrocytes treated with IL-1β for 72 hours. Changes in the number of receptors are shown by the shift in the fluorescence (clear histograms, solid lines) to the left as compared to the corresponding non-stimulated culture control (shaded histograms); dotted lines reflect the background fluorescence produced by the secondary antibody only (negative control). E-H, Linear graphs for each receptor separately represent the mean florescence intensity of seven independent experiments (mean ± SD); in each case ▴ represent cells treated with IL-1β; □ indicates corresponding non-stimulated control. The data were corrected for the negative background fluorescence caused by secondary antibody at each time point.
Figure 4. Phosphorylation of R-Smads at the BMP-specific C-terminal motif (P-Smad) or within the linker region (P-PSSA) by OP-1, IL-1β, and the sequential treatment with IL-1β and OP-1
A, representative gels of chondrocytes treated with either OP-1 only for 4 hours, IL-1β only for 30 minutes replaced with fresh media contained no IL-1β, or chondrocytes pre-treated for 30 minutes with IL-1β followed by the treatment with OP-1 for another 4 hours. T-R-Smad, representative control gel with the antibody against total non-phosphorylated Smad1, where the same samples were used as in gels with combined treatment. Western blot with the antibody against total Smad1 (as a control) was performed separately for each treatment group and is similar in appearance to the blots with total Smad 5 or 8. B, Densitometry of gels stained with P-Smad antibody of chondrocytes cultured in the presence of OP-1 only or combined IL-1β and OP-1. The histograms represent the relative amounts of phosphosmad protein levels from densitometric scanning of immunoblots (mean ± SD) from three independent experiments normalized to total Smad1.
Figure. 5. The mechanism of linker region phosphorylation
A, Human articular chondrocytes were pre-incubated for 1 hour with specific inhibitors of MAP kinases, JNK, ERK, and p38, prior to stimulation with bFGF, IL-1β, or OP-1; all for 1 hour. Cell lysates were blotted with PSSA antibody or antibody against total Smad1 (as a control). Western blotting with anti-total Smad1 antibody was performed for each experimental condition separately. B, Western blot with the antibody against phosphorylated p38 kinase (IL-1β control). Chondrocytes treated with IL-1β for 2 hours and collected at 5, 15, 30, 45, 60, 90, and 120 minutes. C, Combined OP-1 and IL-1β treatment. Chondrocytes pre-treated with OP-1 for 1 hour followed by the addition of IL-1β for 2 hours (both factors were present together in culture). Sample collection was at 1 hour of OP-1 treatment and then at 5, 15, 30, 45, 60, 90, and 120 minutes of combined treatment. Cell lysates were immunoblotted with antibodies against phosphorylated p38 (p-p38; upper panel) and Smad linker region (p-PSSA; bottom panel). As a control, cell lysates were blotted with an antibody for non-phosphorylated p38 (middle panel). D, Phosphorylation of R-Smad at C-terminal motif (P-Smad) by combined IL-1β and OP-1. Chondrocytes were pre-treated with p38 inhibitor (20 μM SB203580) for 1 hour and IL-1β for 30 min (total 1.5 hours) before the media was replaced with fresh media contained OP-1. Cells were cultured in the presence of OP-1 for 4 hours and collected according to schema used in Figure 4. Cell lysates were immunoblotted with the antibodies against phosphorylated (BMP-mediated) and total Smad1. E. Restoration of PG synthesis by p38 inhibitor. Experimental design is the same as in Fig. 1. Chondrocytes were pretreated with either IL-1β alone or in combination with p38 inhibitor for 24 hours, then the media were replaced with the fresh media contained OP-1 for another 48 hours. The data are presented as CPM normalized to the cellular DNA content. The histograms represent the mean ± SD of 2 independent experiments.
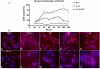
Figure 6. Effect of IL-1β on OP-1 induced Smad4 nuclear translocation
Chondrocytes were stimulated with OP-1 ( ), IL-1β (o) alone or pre-treated with IL-1β for 30 min followed by the treatment with OP-1 (Δ) according to general design of the study. A, Graphical representation of the percentage of chondrocytes contained fluorescently labeled Smad4 in the nuclei (red dots over the blue DAPI stain) at each time point under each treatment. The data presented as the mean ± SD from four independent experiments. B, Representative photomicrographs of Smad 4 translocation induced by either OP-1 alone from 0 min to 4 h (upper panel), IL-1β alone for 30 min (control; lower panel, f) or by combined IL-1β+OP-1 (lower panel, g-j). a, media control, 0 min; b, chondrocytes stimulated with OP-1 for 30 minutes; c, chondrocytes stimulated with OP-1 for 1 hour; d, chondrocytes stimulated with OP-1 for 2 hours; e, chondrocytes stimulated with OP-1 for 4 hours; f, IL-1β control; g, chondrocytes pre-treated with IL-1β for 30 minutes and cultured in the presence of OP-1 for 30 minutes; h, chondrocytes pre-treated with IL-1β for 30 minutes and cultured in the presence of OP-1 for 1 hour; i, chondrocytes pre-treated with IL-1β for 30 minutes and cultured in the presence of OP-1 for 2 hours; j, chondrocytes pre-treated with IL-1β for 30 minutes and cultured in the presence of OP-1 for 4 hours.
Similar articles
- PKC-δ Promotes IL-1β-Induced Apoptosis of Rat Chondrocytes and Via Activating JNK and P38 MAPK Pathways.
Lu J, Yu M, Li J. Lu J, et al. Cartilage. 2024 Sep;15(3):315-327. doi: 10.1177/19476035231181446. Epub 2023 Jul 25. Cartilage. 2024. PMID: 37491820 Free PMC article. - Toll-like receptors and chondrocytes: the lipopolysaccharide-induced decrease in cartilage matrix synthesis is dependent on the presence of toll-like receptor 4 and antagonized by bone morphogenetic protein 7.
Bobacz K, Sunk IG, Hofstaetter JG, Amoyo L, Toma CD, Akira S, Weichhart T, Saemann M, Smolen JS. Bobacz K, et al. Arthritis Rheum. 2007 Jun;56(6):1880-93. doi: 10.1002/art.22637. Arthritis Rheum. 2007. PMID: 17530716 - Bone morphogenetic proteins.
Chen D, Zhao M, Mundy GR. Chen D, et al. Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review. - Roles of SMAD and SMAD-Associated Signaling Pathways in Nerve Regeneration Following Peripheral Nerve Injury: A Narrative Literature Review.
Lee J, Yon DK, Choi YS, Lee J, Yeo JH, Kim SS, Lee JM, Yeo SG. Lee J, et al. Curr Issues Mol Biol. 2024 Jul 22;46(7):7769-7781. doi: 10.3390/cimb46070460. Curr Issues Mol Biol. 2024. PMID: 39057101 Free PMC article. Review.
Cited by
- BMP5 silencing inhibits chondrocyte senescence and apoptosis as well as osteoarthritis progression in mice.
Shao Y, Zhao C, Pan J, Zeng C, Zhang H, Liu L, Fan K, Liu X, Luo B, Fang H, Bai X, Zhang H, Cai D. Shao Y, et al. Aging (Albany NY). 2021 Mar 19;13(7):9646-9664. doi: 10.18632/aging.202708. Epub 2021 Mar 19. Aging (Albany NY). 2021. PMID: 33744859 Free PMC article. - TGFβ/BMP Signaling Pathway in Cartilage Homeostasis.
Thielen NGM, van der Kraan PM, van Caam APM. Thielen NGM, et al. Cells. 2019 Aug 24;8(9):969. doi: 10.3390/cells8090969. Cells. 2019. PMID: 31450621 Free PMC article. Review. - Aging and oxidative stress reduce the response of human articular chondrocytes to insulin-like growth factor 1 and osteogenic protein 1.
Loeser RF, Gandhi U, Long DL, Yin W, Chubinskaya S. Loeser RF, et al. Arthritis Rheumatol. 2014 Aug;66(8):2201-9. doi: 10.1002/art.38641. Arthritis Rheumatol. 2014. PMID: 24664641 Free PMC article. - Osteoarthritis-Related Inflammation Blocks TGF-β's Protective Effect on Chondrocyte Hypertrophy via (de)Phosphorylation of the SMAD2/3 Linker Region.
Thielen N, Neefjes M, Wiegertjes R, van den Akker G, Vitters E, van Beuningen H, Blaney Davidson E, Koenders M, van Lent P, van de Loo F, van Caam A, van der Kraan P. Thielen N, et al. Int J Mol Sci. 2021 Jul 29;22(15):8124. doi: 10.3390/ijms22158124. Int J Mol Sci. 2021. PMID: 34360888 Free PMC article. - Rehabilitation and return-to-sports activity after debridement and bone marrow stimulation of osteochondral talar defects.
van Eekeren IC, Reilingh ML, van Dijk CN. van Eekeren IC, et al. Sports Med. 2012 Oct 1;42(10):857-70. doi: 10.1007/BF03262299. Sports Med. 2012. PMID: 22963224 Review.
References
- Chambers MG, Bayliss MT, Mason RM. Chondrocyte cytokine and growth factor expression in murine osteoarthritis. Osteoarthritis Cartilage. 1997;5:301–8. - PubMed
- Wozney JM, Rosen V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin. Orthop. Relat. Res. 1998:26–37. - PubMed
- Flechtenmacher J, Huch K, Thonar EJ, Mollenhauer JA, Davies SR, Schmid TM, et al. Recombinant human osteogenic protein 1 is a potent stimulator of the synthesis of cartilage proteoglycans and collagens by human articular chondrocytes. Arthritis Rheum. 1996;39:1896–904. - PubMed
- Nishida Y, Knudson CB, Eger W, Kuettner KE, Knudson W. Osteogenic protein 1 stimulates cells-associated matrix assembly by normal human articular chondrocytes: up-regulation of hyaluronan synthase, CD44, and aggrecan. Arthritis Rheum. 2000;43:206–14. - PubMed
- Huch K, Wilbrink B, Flechtenmacher J, Koepp HE, Aydelotte MB, Sampath TK, et al. Effects of recombinant human osteogenic protein 1 on the production of proteoglycan, prostaglandin E2, and interleukin-1 receptor antagonist by human articular chondrocytes cultured in the presence of interleukin-1beta. Arthritis Rheum. 1997;40:2157–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR-39239/AR/NIAMS NIH HHS/United States
- R01 AR047654/AR/NIAMS NIH HHS/United States
- P50 AR039239/AR/NIAMS NIH HHS/United States
- R01 AR047654-04/AR/NIAMS NIH HHS/United States
- AR-47654/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous