Disruption of a Plasmodium falciparum multidrug resistance-associated protein (PfMRP) alters its fitness and transport of antimalarial drugs and glutathione - PubMed (original) (raw)
Disruption of a Plasmodium falciparum multidrug resistance-associated protein (PfMRP) alters its fitness and transport of antimalarial drugs and glutathione
Dipak Kumar Raj et al. J Biol Chem. 2009.
Abstract
ATP-binding cassette transporters play an important role in drug resistance and nutrient transport. In the human malaria parasite Plasmodium falciparum, a homolog of the human p-glycoprotein (PfPgh-1) was shown to be involved in resistance to several drugs. More recently, many transporters were associated with higher IC(50) levels in responses to chloroquine (CQ) and quinine (QN) in field isolates. Subsequent studies, however, could not confirm the associations, although inaccuracy in drug tests in the later studies could contribute to the lack of associations. Here we disrupted a gene encoding a putative multidrug resistance-associated protein (PfMRP) that was previously shown to be associated with P. falciparum responses to CQ and QN. Parasites with disrupted PfMRP (W2/MRPDelta) could not grow to a parasitemia higher than 5% under normal culture conditions, possibly because of lower efficiency in removing toxic metabolites. The W2/MRPDelta parasite also accumulated more radioactive glutathione, CQ, and QN and became more sensitive to multiple antimalarial drugs, including CQ, QN, artemisinin, piperaquine, and primaquine. PfMRP was localized on the parasite surface membrane, within membrane-bound vesicles, and along the straight side of the D-shaped stage II gametocytes. The results suggest that PfMRP plays a role in the efflux of glutathione, CQ, and QN and contributes to parasite responses to multiple antimalarial drugs, possibly by pumping drugs outside the parasite.
Figures
FIGURE 1.
Genetic knock-out of PfMRP in W2 P. falciparum parasite. A, Kyte_Doolittle hydrophilicity plot of the predicted amino acid sequence of PfMRP, showing two regions (horizontal bars) used in DNA vaccination to generate antibodies against PfMRP. The x axis is amino acid position, and the y axis is the hydrophilicity score.B, diagram showing a plasmid construct used to disrupt the gene encoding PfMRP. hdhfr is the gene encoding human dihydrofolate reductase, and amp is the gene encoding ampicillin resistance protein. The arrowheads (F1 and R1) indicate PCR primer positions before and after integration of the plasmid into chromosome. Restriction sites and predicted PCR product sizes are as marked. C, PCR products amplified from wild type W2 and W2/MRPΔ parasites using primers F1 and R1 in B. MW, molecular weight markers. The sizes of the PCR products were as expected from DNA sequences with or without plasmid integration, respectively. D, Western blot showing a 214-kDa band in W2 but not in W2/MRPΔ using mouse anti-PfMRP antibodies; no bands were detected in both W2 and W2/MRPΔ using mouse preimmune sera. Anti-PfCRT was used as loading control.
FIGURE 2.
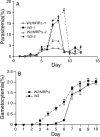
Impaired asexual growth and changes in sexual development in the W2/MRPΔ parasite. A, synchronized wild type W2 and W2/MRPΔ parasites were diluted to 0.1% parasitemia and allowed to grow with change of media once or twice a day (without the addition of fresh red blood cells). W2/MRPΔ-1 and W2–1 were grown in culture with medium change once a day. W2/MRPΔ-2 and W2–2 were the same parasites, but grown in cultures with medium changes twice a day. Parasite cultures “crashed” after day 8 or 9, particularly for those of wild type parasites. B, gametocytemia from W2 and W2/MRPΔ parasites; stage II–V gametocytes were counted daily. The data points in the figures were averages from three independent experiments, and the_bars_ indicate standard deviations.
FIGURE 3.
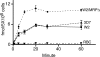
GSH accumulation assays. Parasitized RBCs or uninfected RBCs were incubated with radioactive GSH (the curves shown are from 3 n
m
GSH), and the radioactivities in the RBCs were measured at different time points. Unincorporated radioactive materials were removed by spinning the RBC through silicon oil. The data were from three independent repeats. Similar accumulation curve patterns were obtained using 1 and 6 n
m
radioactive labeled GSH (data not shown).
FIGURE 4.
CQ and QN accumulation assays with or without GSH. A, CQ accumulation. B, CQ radioactivity at 30 min. C, QN accumulation. D, QN radioactivity at 30 min. Parasites and treatments with or without GSH are as indicated. The standard deviations were from three independent experiments.
FIGURE 5.
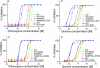
Changes in responses to CQ and QN at the presence of exogenous GSH (2.5 mm) and GSSG (5.0 mm). A and C, response to CQ. B and D, response to QN. The parasite groups used are as indicated in the figures. The data presented were averages from three independent repeats. The graphs were plotted using a nonlinear regression model in GraphPad Prism 5 (La Jolla, CA) with constraint at the top of the graphs but no constraint at the bottom.
FIGURE 6.
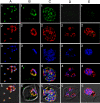
Expression and localization of PfMRP within asexual stages. Row 1, green, anti-PfMRP; row 2, red, anti-PfMSP-1; row 3, blue, 4′,6′-diamino-2-phenylindole dye; row 4, merged rows 1–3; row 5, merged rows 1–4 and differential interference contrast. A, free merozoites of the W2 parasite. B, a trophozoite of the W2 parasite. C, a schizont of the W2 parasite. D, three trophozoites of the W2/MRPΔ parasites within a red blood cell. E, a schizont of the W2/MRPΔ parasite.
FIGURE 7.
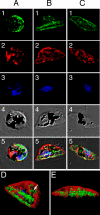
Localization of PfMRP in sexual stages. Row 1, green, anti-PfMRP; row 2, red, anti-PfMDV-1; row 3, blue, 4′,6′-diamino-2-phenylindole dye; row 4, differential interference contrast; row 5, merged rows 1–4. A, stage I gametocyte identified by anti-PfMDV-1 staining. B, stage II gametocyte showing two broad straight lines stained strongly with anti-PfMRP.C, stage III–V gametocyte showing surface membrane staining and some internal membrane networks. D and E, iso-surface model of the stage II gametocyte reconstructed from serial Z-sections of confocal images. D, side view of a stage II gametocyte showing two heavily stained structures (green) parallel to the straight side of the D-shaped parasite. Internal vesicles with PfMRP (white arrowhead) could also be seen inside the parasite. E, bottom view of the same stage II gametocyte. The red shown in this transparent iso-surface model indicates the distribution of PfMDV-1 that is a gametocyte-specific protein located on gametocyte outside membrane and internal vesicles (33).
FIGURE 8.
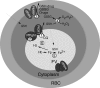
Proposed roles of GSH and PfMRP in CQ and other drug resistances in_P. falciparum_. 1, PfCRT (chloroquine-resistant transporter) plays a key role in CQ resistance, possibly by transporting CQ out of FV. 2, PfPgh-1 may interact with PfCRT and modulate the physiology of FV, which may affect CQ transport. 3, CQ (and quinine) is pumped out of parasite through PfMRP and other molecules, possibly as drug-GSH conjugates. 4, GSH can help remove oxygen radicals such as H2O2. GSSG could be transported out by PfMRP and other transporters. 5, GSH was reported to degrade heme (Fp), a side product from hemoglobin (Hb) digestion. 6, CQ was proposed to inhibit polymerization of Fp into hemozoin (Hz).
Similar articles
- Multiple transporters associated with malaria parasite responses to chloroquine and quinine.
Mu J, Ferdig MT, Feng X, Joy DA, Duan J, Furuya T, Subramanian G, Aravind L, Cooper RA, Wootton JC, Xiong M, Su XZ. Mu J, et al. Mol Microbiol. 2003 Aug;49(4):977-89. doi: 10.1046/j.1365-2958.2003.03627.x. Mol Microbiol. 2003. PMID: 12890022 - Atorvastatin as a potential anti-malarial drug: in vitro synergy in combinational therapy with quinine against Plasmodium falciparum.
Parquet V, Henry M, Wurtz N, Dormoi J, Briolant S, Gil M, Baret E, Amalvict R, Rogier C, Pradines B. Parquet V, et al. Malar J. 2010 May 25;9:139. doi: 10.1186/1475-2875-9-139. Malar J. 2010. PMID: 20497586 Free PMC article. - Monitoring of in vitro susceptibilities and molecular markers of resistance of Plasmodium falciparum isolates from Thai-Myanmar border to chloroquine, quinine, mefloquine and artesunate.
Chaijaroenkul W, Wisedpanichkij R, Na-Bangchang K. Chaijaroenkul W, et al. Acta Trop. 2010 Feb;113(2):190-4. doi: 10.1016/j.actatropica.2009.10.016. Epub 2009 Oct 30. Acta Trop. 2010. PMID: 19879850 - Inhibition of efflux of quinolines as new therapeutic strategy in malaria.
Henry M, Alibert S, Rogier C, Barbe J, Pradines B. Henry M, et al. Curr Top Med Chem. 2008;8(7):563-78. doi: 10.2174/156802608783955593. Curr Top Med Chem. 2008. PMID: 18473883 Review. - Transporters involved in resistance to antimalarial drugs.
Valderramos SG, Fidock DA. Valderramos SG, et al. Trends Pharmacol Sci. 2006 Nov;27(11):594-601. doi: 10.1016/j.tips.2006.09.005. Epub 2006 Sep 25. Trends Pharmacol Sci. 2006. PMID: 16996622 Free PMC article. Review.
Cited by
- Fitness cost of resistance for lumefantrine and piperaquine-resistant Plasmodium berghei in a mouse model.
Gimode WR, Kiboi DM, Kimani FT, Wamakima HN, Burugu MW, Muregi FW. Gimode WR, et al. Malar J. 2015 Jan 28;14:38. doi: 10.1186/s12936-015-0550-5. Malar J. 2015. PMID: 25627576 Free PMC article. - Role and Regulation of Glutathione Metabolism in Plasmodium falciparum.
Müller S. Müller S. Molecules. 2015 Jun 8;20(6):10511-34. doi: 10.3390/molecules200610511. Molecules. 2015. PMID: 26060916 Free PMC article. Review. - Mechanisms of in vitro resistance to dihydroartemisinin in Plasmodium falciparum.
Cui L, Wang Z, Miao J, Miao M, Chandra R, Jiang H, Su XZ, Cui L. Cui L, et al. Mol Microbiol. 2012 Oct;86(1):111-28. doi: 10.1111/j.1365-2958.2012.08180.x. Epub 2012 Aug 6. Mol Microbiol. 2012. PMID: 22812578 Free PMC article. - Identification of a mutant PfCRT-mediated chloroquine tolerance phenotype in Plasmodium falciparum.
Valderramos SG, Valderramos JC, Musset L, Purcell LA, Mercereau-Puijalon O, Legrand E, Fidock DA. Valderramos SG, et al. PLoS Pathog. 2010 May 13;6(5):e1000887. doi: 10.1371/journal.ppat.1000887. PLoS Pathog. 2010. PMID: 20485514 Free PMC article. - Discovery, mechanisms of action and combination therapy of artemisinin.
Cui L, Su XZ. Cui L, et al. Expert Rev Anti Infect Ther. 2009 Oct;7(8):999-1013. doi: 10.1586/eri.09.68. Expert Rev Anti Infect Ther. 2009. PMID: 19803708 Free PMC article. Review.
References
- Dean, M., and Annilo, T. (2005) Annu. Re.v Genomics Hum. Genet. 6 123-142 - PubMed
- Igarashi, Y., Aoki, K. F., Mamitsuka, H., Kuma, K., and Kanehisa, M. (2004) Mol. Biol. Evol. 21 2149-2160 - PubMed
- Stefkova, J., Poledne, R., and Hubacek, J. A. (2004) Physiol. Res. 53 235-243 - PubMed
- Dean, M. (2005) Methods Enzymol. 400 409-429 - PubMed
- Ernst, R., Klemm, R., Schmitt, L., and Kuchler, K. (2005) Methods Enzymol. 400 460-484 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases