Distinct roles for mammalian target of rapamycin complexes in the fibroblast response to transforming growth factor-beta - PubMed (original) (raw)
Distinct roles for mammalian target of rapamycin complexes in the fibroblast response to transforming growth factor-beta
Rod A Rahimi et al. Cancer Res. 2009.
Abstract
Transforming growth factor-beta (TGF-beta) promotes a multitude of diverse biological processes, including growth arrest of epithelial cells and proliferation of fibroblasts. Although the TGF-beta signaling pathways that promote inhibition of epithelial cell growth are well characterized, less is known about the mechanisms mediating the positive response to this growth factor. Given that TGF-beta has been shown to promote fibrotic diseases and desmoplasia, identifying the fibroblast-specific TGF-beta signaling pathways is critical. Here, we investigate the role of mammalian target of rapamycin (mTOR), a known effector of phosphatidylinositol 3-kinase (PI3K) and promoter of cell growth, in the fibroblast response to TGF-beta. We show that TGF-beta activates mTOR complex 1 (mTORC1) in fibroblasts but not epithelial cells via a PI3K-Akt-TSC2-dependent pathway. Rapamycin, the pharmacologic inhibitor of mTOR, prevents TGF-beta-mediated anchorage-independent growth without affecting TGF-beta transcriptional responses or extracellular matrix protein induction. In addition to mTORC1, we also examined the role of mTORC2 in TGF-beta action. mTORC2 promotes TGF-beta-induced morphologic transformation and is required for TGF-beta-induced Akt S473 phosphorylation but not mTORC1 activation. Interestingly, both mTOR complexes are necessary for TGF-beta-mediated growth in soft agar. These results define distinct and overlapping roles for mTORC1 and mTORC2 in the fibroblast response to TGF-beta and suggest that inhibitors of mTOR signaling may be useful in treating fibrotic processes, such as desmoplasia.
Figures
Figure 1. TGF-β activates mTORC1 in fibroblasts but not epithelial cells
A, AKR-2B fibroblasts (left panel) or Mv1Lu epithelial cells (right panel) were serum-starved overnight and stimulated with 5 ng/ml TGF-β. At the indicated times, Western analysis was performed using antibodies to phospho (pS6K1 T389 and pSmad2) or total S6K1 and Smad2. B, Three fibroblast (AKR-2B, Swiss3T3, and IMR-90) and three epithelial (Mv1Lu, MDCK, and Hela) cell lines were treated as above and stimulated with TGF-β for 4 hours. Western blot analysis was performed as in panel A.
Figure 2. TGF-β activates mTORC1 via a PI3K-Akt-TSC2 dependent pathway
A, AKR-2B cells were serum-starved overnight followed by pretreatment with vehicle (0.1% DMSO), LY294002 (10 μM), UO126 (10 μM), or Rapamycin (10 nM) for 30 minutes. Cells were left untreated (-) or stimulated (+) with TGF-β (5 ng/ml) for 4 hours in the presence of the indicated inhibitors. Western blot analysis was performed as described in Materials and Methods. B, AKR-2B fibroblasts were prepared as in panel A and stimulated with 5 ng/ml TGF-β for the indicated times. Western analysis was performed using antibodies to phospho (pTSC2 S939 and pAkt S473) or total TSC2 and Akt. C, TSC2 -/- MEFs were transfected with HA-S6K1 and TSC2 wild type (WT) or TSC2 SATA. Cells were serum-starved overnight and subsequently stimulated with 5 ng/ml TGF-β for 4 hours. HA-S6K1 was immunoprecipitated and assayed for phosphorylation (pS6K1 T389) or total expression (Anti-HA). Western blot analysis was performed on total cell lysate for FLAG-TSC2, phospho-Smad2 (pSmad2), and total Smad2. D, AKR-2B cells were serum-starved overnight followed by pretreatment with vehicle (H2O) or CHX (cycloheximide, 1.25 ug/ml) for 30 minutes. Cells were subsequently stimulated with TGF-β for 6 hours. Western blot analysis performed as described in Materials and Methods.
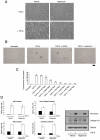
Figure 3. Rapamycin inhibits TGF-β mediated anchorage-independent growth of AKR-2B cells
A, AKR-2B fibroblasts were serum-starved overnight and subsequently pretreated with either vehicle (0.1% EtOH) or 10 nM rapamycin for 30 minutes. Cells were then left untreated (-) or stimulated (+) with 5 ng/ml TGF-β for 48 hours. Photographs of representative fields are shown. B, AKR-2B cells were seeded in soft agar with the indicated treatments as described in Materials and Methods. Photographs of representative fields are shown following 10 days growth. Trypan blue exclusion showed that rapamycin did not increase cell death over the course of seven days (data not shown). Scale bar (far right) represents 100 μm. C, Quantitation of AKR-2B soft agar experiments performed with indicated treatments. Data represent the mean ± standard error of 3 independent experiments each done in triplicate. D, AKR-2B cells were transfected with ARE, SBE, Type I collagen, or Fibronectin responsive luciferase reporter constructs, serum-starved overnight, and subsequently pretreated with either vehicle (0.1% EtOH) or 10 nM rapamycin for 30 minutes. Cells were then left untreated (black) or stimulated (white) with 5 ng/ml TGF-β for 24 hours. Relative lights units are presented with all values relative to unstimulated control cells. Mean ± standard error of 3 independent experiments performed in triplicate are shown. Asterisk represents statistical significance (p<0.05) compared to control (vehicle and TGF-β treated) using an independent two sample t-test. D (right panel), AKR-2B fibroblasts were serum-starved overnight and subsequently pretreated with either vehicle (0.1% EtOH) or 10 nM rapamycin for 30 minutes. Cells were then left untreated (-) or stimulated (+) with 5 ng/ml TGF-β for 24 hours. Western analysis was performed to determine the protein expression of fibronectin and Collagen1A1. GAPDH was used as a loading control.
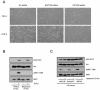
Figure 4. mTORC2 is required for TGF-β mediated Akt S473 phosphorylation but not mTORC1 signaling
A, mLST8 +/- and mLST8 -/- MEFs were serum-starved overnight and either left untreated (-) or stimulated (+) with 5 ng/ml TGF-β for 4 hours. Western blots were performed with indicated phospho and corresponding total antibodies. B, Stable AKR-2B cell lines expressing either Non-targeting (NT), RAPTOR targeting, or RICTOR targeting shRNA were serum-starved overnight and either left untreated (-) or stimulated (+) with 5 ng/ml TGF-β for 6 hours and processed for Western analysis with indicated antibodies.
Figure 5. mTORC2 is required for TGF-β morphologic transformation and is insensitive to long-term rapamycin in fibroblast cell lines
A, The effect of Non-targeting (NT), RAPTOR targeting, or RICTOR targeting shRNA on AKR-2B morphologic transformation was determined as discussed in Fig. 3A and Materials and Methods. Photographs of representative fields are shown. B, AKR-2B cells were serum-starved for 24 hours in the presence of 0.1% EtOH or 10 nM rapamycin. Cells were then left untreated or stimulated with TGF-β (5 ng/ml) for 4 hours. Western analysis was performed using antibodies to phospho (pAkt S473 and pS6K1 T389) or total Akt and S6K1. C, Three fibroblast (AKR-2B, Swiss3T3, and IMR-90) cell lines were grown in DMEM supplemented with 10% serum in the presence of 0.1% EtOH (-) or 10 nM rapamycin (+) for 24 hours. Western analysis was performed with indicated antibodies on proliferating cultures.
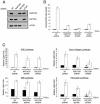
Figure 6. mTORC1 and mTORC2 have distinct roles in the fibroblast response to TGF-β
A, AKR-2B cells were transiently transduced with lentiviruses expressing either Non-targeting (NT), RAPTOR targeting, RICTOR targeting, or mTOR targeting shRNAs. 72 hours post-transduction cells were lysed and Western blots performed with indicated antibodies to show loss of the specific protein targeted. B, AKR-2B cells were transduced with lentivirus as in panel A and 72 hours post-transduction were seeded in soft agar with (white) or without (black) 5 ng/ml TGF-β. Quantitation of colony formation after 10 days of growth is shown. Data represent mean ± standard error from 3 experiments done in triplicate. C, AKR-2B cell lines stably expressing Non-targeting (NT), RAPTOR targeting, or RICTOR targeting shRNAs were transiently transfected with ARE, SBE, Type I collagen, or Fibronectin responsive luciferase reporter constructs, serum-starved overnight, and left untreated (black) or stimulated (white) with 5 ng/ml TGF-β for 24 hours. Relative lights units are presented with all values relative to unstimulated control cells. Mean ± standard error relative to unstimulated control cells of 3-4 independent experiments done in triplicate are shown. The fold induction values of the Type I collagen reporter for NT shRNA, RAPTOR shRNA, and RICTOR shRNA are 5.8±1.0, 4.2±0.9, 8.0±0.8. The fold induction values of the FN reporter for NT shRNA, RAPTOR shRNA, and RICTOR shRNA are 6.8±1.3, 3.5±0.6, 5.5±0.7. Asterisk and pound symbols represent statistical significance (p<0.05) difference in relative light units compared to control cells using an independent two sample t-test. * is compared to NT shRNA cells treated with TGF-β while # is compared to untreated NT shRNA cells.
Similar articles
- A critical role for the mTORC2 pathway in lung fibrosis.
Chang W, Wei K, Ho L, Berry GJ, Jacobs SS, Chang CH, Rosen GD. Chang W, et al. PLoS One. 2014 Aug 27;9(8):e106155. doi: 10.1371/journal.pone.0106155. eCollection 2014. PLoS One. 2014. PMID: 25162417 Free PMC article. - TGF-β Promotes Metabolic Reprogramming in Lung Fibroblasts via mTORC1-dependent ATF4 Activation.
O'Leary EM, Tian Y, Nigdelioglu R, Witt LJ, Cetin-Atalay R, Meliton AY, Woods PS, Kimmig LM, Sun KA, Gökalp GA, Mutlu GM, Hamanaka RB. O'Leary EM, et al. Am J Respir Cell Mol Biol. 2020 Nov;63(5):601-612. doi: 10.1165/rcmb.2020-0143OC. Am J Respir Cell Mol Biol. 2020. PMID: 32668192 Free PMC article. - Signaling events downstream of mammalian target of rapamycin complex 2 are attenuated in cells and tumors deficient for the tuberous sclerosis complex tumor suppressors.
Huang J, Wu S, Wu CL, Manning BD. Huang J, et al. Cancer Res. 2009 Aug 1;69(15):6107-14. doi: 10.1158/0008-5472.CAN-09-0975. Epub 2009 Jul 14. Cancer Res. 2009. PMID: 19602587 Free PMC article. - A complex interplay between Akt, TSC2 and the two mTOR complexes.
Huang J, Manning BD. Huang J, et al. Biochem Soc Trans. 2009 Feb;37(Pt 1):217-22. doi: 10.1042/BST0370217. Biochem Soc Trans. 2009. PMID: 19143635 Free PMC article. Review. - Mammalian target of rapamycin and tuberous sclerosis complex.
Wataya-Kaneda M. Wataya-Kaneda M. J Dermatol Sci. 2015 Aug;79(2):93-100. doi: 10.1016/j.jdermsci.2015.04.005. Epub 2015 Apr 25. J Dermatol Sci. 2015. PMID: 26051878 Review.
Cited by
- Autophagy in idiopathic pulmonary fibrosis.
Patel AS, Lin L, Geyer A, Haspel JA, An CH, Cao J, Rosas IO, Morse D. Patel AS, et al. PLoS One. 2012;7(7):e41394. doi: 10.1371/journal.pone.0041394. Epub 2012 Jul 18. PLoS One. 2012. PMID: 22815997 Free PMC article. - NK Cell Metabolism and TGFβ - Implications for Immunotherapy.
Slattery K, Gardiner CM. Slattery K, et al. Front Immunol. 2019 Dec 13;10:2915. doi: 10.3389/fimmu.2019.02915. eCollection 2019. Front Immunol. 2019. PMID: 31921174 Free PMC article. Review. - Non-Smad transforming growth factor-β signaling regulated by focal adhesion kinase binding the p85 subunit of phosphatidylinositol 3-kinase.
Hong M, Wilkes MC, Penheiter SG, Gupta SK, Edens M, Leof EB. Hong M, et al. J Biol Chem. 2011 May 20;286(20):17841-50. doi: 10.1074/jbc.M111.233676. Epub 2011 Mar 28. J Biol Chem. 2011. PMID: 21454615 Free PMC article. - Calpain-2 activates Akt via TGF-β1-mTORC2 pathway in pulmonary artery smooth muscle cells.
Abeyrathna P, Kovacs L, Han W, Su Y. Abeyrathna P, et al. Am J Physiol Cell Physiol. 2016 Jul 1;311(1):C24-34. doi: 10.1152/ajpcell.00295.2015. Epub 2016 Apr 20. Am J Physiol Cell Physiol. 2016. PMID: 27099352 Free PMC article. - From TOR and SMAD, why HIF-1α can be bad.
Sheikh-Hamad D. Sheikh-Hamad D. Am J Physiol Renal Physiol. 2014 Jan;306(2):F170-1. doi: 10.1152/ajprenal.00486.2013. Epub 2013 Sep 18. Am J Physiol Renal Physiol. 2014. PMID: 24049143 Free PMC article. No abstract available.
References
- Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–21. - PubMed
- Rahimi RA, Leof EB. TGF-beta signaling: A tale of two responses. J Cell Biochem. 2007;102:593–608. - PubMed
- Wilkes MC, Mitchell H, Gulati-Penheiter S, et al. Transforming growth factor-b activation of phosphatidylinositol 3-kinase is independent of Smad2 and Smad3 and regulates fibroblast responses via p21-activated kinase-2. Cancer Res. 2005;65:10431–40. - PubMed
- Evans RA, Tian YC, Steadman R, Phillips AO. TGF-beta1-mediated fibroblast-myofibroblast terminal differentiation--the role of smad proteins. Exp Cell Res. 2003;282:90–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM 54200/GM/NIGMS NIH HHS/United States
- R01 GM055816-12/GM/NIGMS NIH HHS/United States
- R01 GM054200-13/GM/NIGMS NIH HHS/United States
- R37 GM055816/GM/NIGMS NIH HHS/United States
- R01 GM055816/GM/NIGMS NIH HHS/United States
- R01 GM054200/GM/NIGMS NIH HHS/United States
- GM 55816/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous