Infection-triggered familial or recurrent cases of acute necrotizing encephalopathy caused by mutations in a component of the nuclear pore, RANBP2 - PubMed (original) (raw)
doi: 10.1016/j.ajhg.2008.12.009.
Mark D Adams, Caitlin M D Orr, Deborah K Schelling, Robert M Eiben, Douglas S Kerr, Jane Anderson, Alexander G Bassuk, Ann M Bye, Anne-Marie Childs, Antonia Clarke, Yanick J Crow, Maja Di Rocco, Christian Dohna-Schwake, Gregor Dueckers, Alfonso E Fasano, Artemis D Gika, Dimitris Gionnis, Mark P Gorman, Padraic J Grattan-Smith, Annette Hackenberg, Alice Kuster, Markus G Lentschig, Eduardo Lopez-Laso, Elysa J Marco, Sotiria Mastroyianni, Julie Perrier, Thomas Schmitt-Mechelke, Serenella Servidei, Angeliki Skardoutsou, Peter Uldall, Marjo S van der Knaap, Karrie C Goglin, David L Tefft, Cristin Aubin, Philip de Jager, David Hafler, Matthew L Warman
Affiliations
- PMID: 19118815
- PMCID: PMC2668029
- DOI: 10.1016/j.ajhg.2008.12.009
Infection-triggered familial or recurrent cases of acute necrotizing encephalopathy caused by mutations in a component of the nuclear pore, RANBP2
Derek E Neilson et al. Am J Hum Genet. 2009 Jan.
Abstract
Acute necrotizing encephalopathy (ANE) is a rapidly progressive encephalopathy that can occur in otherwise healthy children after common viral infections such as influenza and parainfluenza. Most ANE is sporadic and nonrecurrent (isolated ANE). However, we identified a 7 Mb interval containing a susceptibility locus (ANE1) in a family segregating recurrent ANE as an incompletely penetrant, autosomal-dominant trait. We now report that all affected individuals and obligate carriers in this family are heterozygous for a missense mutation (c.1880C-->T, p.Thr585Met) in the gene encoding the nuclear pore protein Ran Binding Protein 2 (RANBP2). To determine whether this mutation is the susceptibility allele, we screened controls and other patients with ANE who are unrelated to the index family. Patients from 9 of 15 additional kindreds with familial or recurrent ANE had the identical mutation. It arose de novo in two families and independently in several other families. Two other patients with familial ANE had different RANBP2 missense mutations that altered conserved residues. None of the three RANBP2 missense mutations were found in 19 patients with isolated ANE or in unaffected controls. We conclude that missense mutations in RANBP2 are susceptibility alleles for familial and recurrent cases of ANE.
Figures
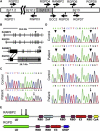
Figure 1
Pedigrees of 16 Families with Familial or Recurrent ANE Pedigrees with mutations in RANBP2 are given in (A) whereas those without mutation are shown in (B). Family numbers are given at top. Symbol notations for (A) as follows: filled, mutation carrier, affected with ANE; filled with white lower right quandrant, mutation carrier, recurrent ANE; vertical bar, mutation carrier; horizontal bar, encephalopathy, unconfirmed as ANE; underlined symbol, DNA studied; circled symbol, molecularly proven de novo mutation carrier. For simplicity, not all siblings and spouses are shown in pedigree 586. Symbol notations for (B) are equivalent to (A) except without mutation.
Figure 2
Mutation Identification in RANBP2 (A) The locations and orientations of RANBP2, GCC2, and the _RGPD_s are shown on a partial ideogram of chromosome 2. (B) A comparison of the genomic regions of RANBP2 and RGPD5 indicating strong structural and sequence conservation for their exons 1-18 (upper lines) and the divergence of their 3′ regions (lower lines). RGPD5 is deleted for a portion of exon 20 and has its final three exons derived from GCC2 (shaded box). This enables _RANBP2_-specific cDNA to be synthesized with a primer (hollow arrow) in the gene's 3′UTR. Also shown is the location of the c.1880C→T mutation in exon 12 of RANBP2 (arrowhead). (C) Sequencing electropherograms of _RANBP2_-specific cDNA reveals a c.1880C→T transition in the patient but not the control. (D) Intronic primers that amplify exon 12 of RANBP2 and the RGPD paralogs (upper two electropherograms) demonstrate multiple paralog-derived signal alterations (arrowheads) in the patient and the control. In contrast, sequence specific to exon 12 of RANBP2 (lower electropherograms) shows the absence of paralog-derived signals in the patient and control, but persistence of the c.1880C→T mutation (arrow) in the patient. (E) Schematic diagram of RANBP2 protein indicating the “leucine-rich” domain (LD), four Ran binding domains (RBD), eight zinc finger repeats (ZnF), a cyclophilin A domain (CyA), and a SUMO E3 ligase domain (E3). In the partial deletion of the 20th exon, the RGPDs lose the ZnF and second RBD domain. The derived exons from GCC2 provide a new C-terminal GRIP domain which targets the RGPDs to the Golgi. The sites of the p.Thr585Met, p.Thr653Ile, and p.Ile656Val LD domain substitutions associated with ANE1 are indicated by arrows.
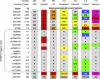
Figure 3
Comparison of Mutation-Bearing Haplotypes in Seven Families with c.1880C→T Mutation The haplotypes of seven families show differences in intragenic and extragenic polymorphic markers, suggesting independent origins for each family of the linked c.1880C→T mutation. Alleles are shown in their contiguous arrangement and represented as STR PCR fragment size (e.g., D2S2229), nucleotide allele (e.g., rs826559), or repeat count (ss76880141). Marker distances are given relative to the c.1880C→T mutation site (0 Kb), with orientation along the plus strand of the chromosome. RANBP2 intragenic markers are delineated between horizontal lines at −4.1 Kb and +25.8 Kb. To highlight the potential for, or lack of, ancestral relationship, matching alleles are given the same unique color. When noninformative, both alleles are shown (e.g., family 102, D2S293).
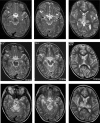
Figure 4
Brain MRI Findings in a Child (690, II:1) with Familial and Recurrent Acute Necrotizing Encephalopathy T2-weighted images obtained during the acute encephalopathy (top) are shown in comparison to resolved images obtained 6 months later (middle) and images from a recurrent episode 3 years later (bottom). During acute events, abnormal signal is visible in the pons (white arrow), midbrain (thin white arrow), amygdala (black arrow), thalamus (black arrowhead), and external capsule (white arrowhead).
Similar articles
- Variable clinical course in acute necrotizing encephalopathy and identification of a novel RANBP2 mutation.
Sell K, Storch K, Hahn G, Lee-Kirsch MA, Ramantani G, Jackson S, Neilson D, von der Hagen M, Hehr U, Smitka M. Sell K, et al. Brain Dev. 2016 Sep;38(8):777-80. doi: 10.1016/j.braindev.2016.02.007. Epub 2016 Feb 26. Brain Dev. 2016. PMID: 26923722 - Recurrent acute necrotizing encephalopathy in a boy with RANBP2 mutation and thermolabile CPT2 variant: The first case of ANE1 in Japan.
Ohashi E, Hayakawa I, Murofushi Y, Kawai M, Suzuki-Muromoto S, Abe Y, Yoshida M, Kono N, Kosaki R, Hoshino A, Mizuguchi M, Kubota M. Ohashi E, et al. Brain Dev. 2021 Sep;43(8):873-878. doi: 10.1016/j.braindev.2021.04.009. Epub 2021 May 28. Brain Dev. 2021. PMID: 34059398 - Familial acute necrotizing encephalopathy with RANBP2 mutation: The first report in Northeast Asia.
Lee YJ, Hwang SK, Lee SM, Kwon S. Lee YJ, et al. Brain Dev. 2017 Aug;39(7):625-628. doi: 10.1016/j.braindev.2017.02.005. Epub 2017 Mar 21. Brain Dev. 2017. PMID: 28336122 Free PMC article. - RANBP2 mutation and acute necrotizing encephalopathy: 2 cases and a literature review of the expanding clinico-radiological phenotype.
Singh RR, Sedani S, Lim M, Wassmer E, Absoud M. Singh RR, et al. Eur J Paediatr Neurol. 2015 Mar;19(2):106-13. doi: 10.1016/j.ejpn.2014.11.010. Epub 2014 Dec 9. Eur J Paediatr Neurol. 2015. PMID: 25522933 Review. - The interplay of infection and genetics in acute necrotizing encephalopathy.
Neilson DE. Neilson DE. Curr Opin Pediatr. 2010 Dec;22(6):751-7. doi: 10.1097/MOP.0b013e3283402bfe. Curr Opin Pediatr. 2010. PMID: 21610332 Review.
Cited by
- Acute necrotizing encephalopathy associated with RANBP2 mutation: value of MRI findings for diagnosis and intervention.
Sarigecili E, Ucar HK, Havali C, Cansu A, Aydin K. Sarigecili E, et al. Acta Neurol Belg. 2023 Apr;123(2):571-582. doi: 10.1007/s13760-022-02166-x. Epub 2022 Dec 26. Acta Neurol Belg. 2023. PMID: 36572756 Free PMC article. - Plasma exchange therapy for acute necrotizing encephalopathy of childhood.
Li K, Zhang T, Liu G, Jin P, Wang Y, Wang L, Xu M, Liu C, Liu Y, Zhou T, Xu Y, Yang Y, Fang B, Yang X, Liu C, Qian S. Li K, et al. Pediatr Investig. 2021 Jun 18;5(2):99-105. doi: 10.1002/ped4.12280. eCollection 2021 Jun. Pediatr Investig. 2021. PMID: 34179705 Free PMC article. - Uncoupling phototoxicity-elicited neural dysmorphology and death by insidious function and selective impairment of Ran-binding protein 2 (Ranbp2).
Cho KI, Haney V, Yoon D, Hao Y, Ferreira PA. Cho KI, et al. FEBS Lett. 2015 Dec 21;589(24 Pt B):3959-68. doi: 10.1016/j.febslet.2015.11.037. Epub 2015 Nov 26. FEBS Lett. 2015. PMID: 26632511 Free PMC article. - Chimeric Genes in Deletions and Duplications Associated with Intellectual Disability.
Mayo S, Monfort S, Roselló M, Orellana C, Oltra S, Caro-Llopis A, Martínez F. Mayo S, et al. Int J Genomics. 2017;2017:4798474. doi: 10.1155/2017/4798474. Epub 2017 May 24. Int J Genomics. 2017. PMID: 28630856 Free PMC article. - Crystal structure of the N-terminal domain of Nup358/RanBP2.
Kassube SA, Stuwe T, Lin DH, Antonuk CD, Napetschnig J, Blobel G, Hoelz A. Kassube SA, et al. J Mol Biol. 2012 Nov 9;423(5):752-65. doi: 10.1016/j.jmb.2012.08.026. Epub 2012 Sep 7. J Mol Biol. 2012. PMID: 22959972 Free PMC article.
References
- Liu R., Paxton W.A., Choe S., Ceradini D., Martin S.R., Horuk R., MacDonald M.E., Stuhlmann H., Koup R.A., Landau N.R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. - PubMed
- Mori S.I., Nagashima M., Sasaki Y., Mori K., Tabei Y., Yoshida Y., Yamazaki K., Hirata I., Sekine H., Ito T. A novel amino acid substitution at the receptor-binding site on the hemagglutinin of H3N2 influenza A viruses isolated from 6 cases with acute encephalopathy during the 1997–1998 season in Tokyo. Arch. Virol. 1999;144:147–155. - PubMed
- Mizuguchi M. Acute necrotizing encephalopathy of childhood: a novel form of acute encephalopathy prevalent in Japan and Taiwan. Brain Dev. 1997;19:81–92. - PubMed
- Mizuguchi M., Yamanouchi H., Ichiyama T., Shiomi M. Acute encephalopathy associated with influenza and other viral infections. Acta Neurol. Scand. 2007;115:45–56. - PubMed
- Mastroyianni S.D., Gionnis D., Voudris K., Skardoutsou A., Mizuguchi M. Acute necrotizing encephalopathy of childhood in non-Asian patients: report of three cases and literature review. J. Child Neurol. 2006;21:872–879. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous