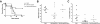Bacterial infection of Smad3/Rag2 double-null mice with transforming growth factor-beta dysregulation as a model for studying inflammation-associated colon cancer - PubMed (original) (raw)
Bacterial infection of Smad3/Rag2 double-null mice with transforming growth factor-beta dysregulation as a model for studying inflammation-associated colon cancer
Lillian Maggio-Price et al. Am J Pathol. 2009 Jan.
Abstract
Alterations in genes encoding transforming growth factor-beta-signaling components contribute to colon cancer in humans. Similarly, mice deficient in the transforming growth factor-beta signaling molecule, Smad3, develop colon cancer, but only after a bacterial trigger occurs, resulting in chronic inflammation. To determine whether Smad3-null lymphocytes contribute to increased cancer susceptibility, we crossed Smad3-null mice with mice deficient in both B and T lymphocytes (Rag2(-/-) mice). Helicobacter-infected Smad3/Rag2-double knockout (DKO) mice had more diffuse inflammation and increased incidence of adenocarcinoma compared with Helicobacter-infected Smad3(-/-) or Rag2(-/-) mice alone. Adoptive transfer of WT CD4(+)CD25(+) T-regulatory cells provided significant protection of Smad3/Rag2-DKO from bacterial-induced typhlocolitis, dysplasia, and tumor development, whereas Smad3(-/-) T-regulatory cells provided no protection. Immunohistochemistry, real-time reverse transcriptase-polymerase chain reaction, and Western blot analyses of colonic tissues from Smad3/Rag2-DKO mice 1 week after Helicobacter infection revealed an influx of macrophages, enhanced nuclear factor-kappaB activation, increased Bcl(XL)/Bcl-2 expression, increased c-Myc expression, accentuated epithelial cell proliferation, and up-regulated IFN-gamma, IL-1alpha, TNF-alpha, IL-1beta, and IL-6 transcription levels. These results suggest that the loss of Smad3 increases susceptibility to colon cancer by at least two mechanisms: deficient T-regulatory cell function, which leads to excessive inflammation after a bacterial trigger; and increased expression of proinflammatory cytokines, enhanced nuclear factor-kappaB activation, and increased expression of both pro-oncogenic and anti-apoptotic proteins that result in increased cell proliferation/survival of epithelial cells in colonic tissues.
Figures
Figure 1
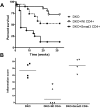
_Helicobacter_-infected DKO mice have decreased survival and increased inflammation and tumor burden compared with Rag_2_KO and Smad3KO mice. A: The Kaplan-Meier survival curve for Smad3/Rag2-DKO (n = 9) mice is significantly less than Rag2KO (n = 11, P = 0.037) and Smad3KO (n = 6, P = 0.03) mice. Mice were euthanized when they developed 20% body weight loss or clinical signs of significant IBD. B: Decreased survival of DKO mice was attributable to differences in inflammation and tumor burden. DKO mice had significantly more inflammation than Rag2KO mice (*P < 0.01) and Smad3KO mice (*P < 0.05). C: Tumor burden scores were considerably higher in DKO mice compared with Rag2KO mice and Smad3KO mice (**P < 0.001). Tumor burden scores represent numbers of only invasive tumors in cecum and colon. Rag2KO mice were Smad3+/−_Rag2_−/−. _Helicobacter_-infected Smad3+/− (n = 5) and WT (n = 10) mice did not develop inflammation or tumors.
Figure 2
_Helicobacter_-infected Smad3/Rag2-DKO mice develop tumors throughout the large bowel compared with localized tumors in _Helicobacter_-infected Smad3KO mice. A: Smad3KO mouse infected with Helicobacter. Tumors are anatomically restricted to the cecocolic junction and proximal colon (arrowheads). The ileum (#) was dissected away and * notes cecum. B: Smad3/Rag2-DKO mouse infected with Helicobacter. The entire colon is markedly thickened with multiple tumors (arrowheads) along the large bowel. Also note the absence of formed feces in the distal colon (arrow). C: Smad3/Rag2-DKO breeder mouse of comparable age maintained _Helicobacter_-free has no gross or histological evidence of tumors.
Figure 3
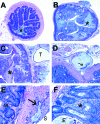
H&E-stained sections of colon from Smad3/Rag2-DKO mice. A: Proximal colon from a _Helicobacter_-free Smad3/Rag2-DKO mouse. Asterisk = lumen. B: Proximal colon from a _Helicobacter_-infected Smad3/Rag2-DKO mouse. Note the markedly thickened mucosa with loss of normal architecture compared with A. Asterisk = lumen. C: Proximal colon from the same animal as B with a large serosal focus of mucinous adenocarcinoma (T). Asterisk = lumen. D: A serosal lesion contains abundant mucous and rafts of neoplastic epithelial cells (arrow). E: Early invasive lesion (arrow). Mucus-producing neoplastic cells (arrow) present within the tunica muscularis with extension out toward the serosa (S). Note hyperplastic glands in the overlying mucosa (M). F: _Helicobacter_-associated hyperplastic colitis in Smad3/Rag2-DKO mice is characterized by elongated and irregular glands with loss of goblet cells, increased mitotic figures with interstitial lymphohistiocytic inflammation, crypt abscesses (asterisk), and glandular ectasia (E), and loss. H&E-stained sections. Original magnifications: ×2 (A, B); ×4 (C); ×10 (D); ×20 (E, F).
Figure 4
Immunohistochemistry of proximal colonic tissue from WT, DKO-broth, and _H. bilis_-infected DKO mice at 1 week after infection. H&E-stained section demonstrating the thin regular normal mucosa of the WT and DKO-broth in contrast to the _H. bilis_-infected DKO where the mucosa is thickened by proliferative epithelium and inflammatory cells. F4/80: Immunohistochemical stain for F4/80 antigen, a macrophage marker. Note the relatively few positive (brown-stained) cells in the WT mouse mucosa. There is a mild increase in positive cells in the DKO-broth colon and a moderate to marked increase in positive signal within the mucosa and submucosa of _H. bilis_-infected DKO mouse. Ki-67: Immunohistochemical stain for Ki-67, a proliferation antigen, is normally restricted to the base of the crypts (brown staining). Note the increased signal in the DKO-H. bilis colon within the crypts, extending lumenally and within the expanded lamina propria. Cleaved caspase-3: Immunohistochemistry for cleaved caspase-3, a marker of apoptosis. In normal colons, apoptotic cells (brown staining) are present at the top of the crypts as a normal progression of cellular turnover. Note increased positive cells within the mucosa of the _H. bilis_-infected DKO mouse, likely reflective of increased cell division. Positive cells (brown staining) are present in increased numbers at the top of the crypt, throughout the length of the crypt, and within luminal contents.
Figure 5
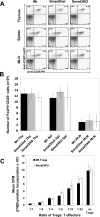
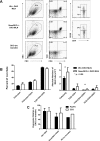
T-regulatory cell numbers and in vitro function in Smad3-null, Smad3-heterozygous, and WT mice. A: Representative flow cytometric profiles showing staining for CD25 and FoxP3 on TCR+CD4+-gated lymphocytes. The profiles indicate similar representation of Treg cells in thymus, spleen, and MLN from WT, Smad3+/−, or _Smad3_−/− animals. B: The average total numbers of TCR+CD4+CD25+FoxP3+ cells in same tissues in A (n = 3 mice of each genotype). C: _Smad_−/− Treg cells are able to suppress proliferation of WT effector T cells in the presence of WT APCs. Treg cells were purified from WT or _Smad3_−/− spleens and set up in assays containing WT effector T cells and APCs (see Materials and Methods for details). Shown is a graph representing the mean cpm of triplicate or duplicate wells at indicated Treg:T-effector ratios. Error bars represent 1 SD of triplicate or duplicate wells using a [3H]thymidine incorporation assay. The assay was repeated twice; once with WT T effectors and APCs and once with Smad3+/− T effectors and APCs. Both assays demonstrated similar results. T cells were stimulated with either concavalin A or anti-CD3ε antibody. Although stimulation with anti-CD3 resulted in less robust proliferative responses, results were similar. An assay using concavalin A stimulation is shown.
Figure 6
Survival and inflammation in Smad3/Rag2-DKO mice receiving adoptively transferred WT or Smad3KO CD4+ cells. A: Kaplan-Meier survival curves for DKO mice adoptively transferred with WT, Smad3KO CD4+ T cells, or no cells (n = 4 per group). DKO mice receiving WT CD4+ cells exhibit a trend of increased survival relative to DKO mice receiving no cells (P = 0.089). Adoptive transfer of Smad3KO CD4+ T cells significantly (P = 0.004) decreased the survival of DKO mice relative to DKO mice receiving no cells. Altered survival curves correlated with differences in inflammation. B: Inflammation was significantly lower (*P < 0.001) in DKO mice receiving WT CD4+ T cells compared with those receiving Smad3KO CD4+ T cells or no cells. Purified WT or Smad3KO CD4+ T cells (4 × 105; 92% pure for both groups) were adoptively transferred into recipient mice by intraperitoneal injection. One month after adoptive transfer, recipients were infected with H. bilis via oral gavage to induce IBD.
Figure 7
Survival and inflammation in Smad3/Rag2-DKO mice receiving WT or Smad3KO CD4+CD25+ T cells. A: Kaplan-Meier survival curves for DKO mice adoptively transferred with WT CD4+CD25+ T cells (n = 12), Smad3KO CD4+CD25+ T cells (n = 5), or no cells (n = 9). DKO mice receiving WT CD4+CD25+ T cells had a significantly better survival curve (P = 0.025) than DKO mice receiving Smad3KO CD4+CD25+ T cells, or no cells. Altered survival curves were related to differences in inflammation. Inflammation (B) and tumor burden (C) were significantly lower (*P < 0.01 for both) in DKO mice receiving WT CD4+CD25+ T cells compared with those receiving Smad3KO CD4+CD25+ T cells, or no cells. These data were compiled from two studies. Sorted cells (3.1 × 105, 97.3% CD4+CD25+ for study 1 or 1.3 × 105, > 98% TCR+CD4+CD25+ for study 2) were adoptively transferred into recipient mice by intraperitoneal injection. Recipients were then infected with H. bilis 5 weeks (study 1) or 2 weeks (study 2) after transfer to induce IBD. Medicated chow was given at 7 days after infection for 9 weeks (study 1) and 5.8 weeks (study 2) and was required to maintain animals in studies.
Figure 8
Adoptively transferred Smad3KO and WT CD4+CD25+ T cells can be detected at comparable levels in DKO recipients. A: Flow cytometric analysis of MLNs from _H. bilis_-infected DKO mice that had received 2 × 105 sorted CD4+CD25+TCR+ splenocytes (>98% pure) from Smad3KO or WT mice. Cells were gated on singlets (not shown), live cells (left), CD4+TCR+ (quadrant, middle), and CD25+ or CD25− (right) for analysis. B: Percentage (left) and absolute numbers (right) of indicated cell types. Averages of values from DKO mice receiving sorted Smad3KO cells (n = 4) and DKO mice receiving sorted WT cells (n = 5) are shown (error bars are 1 SD). C: RNA was isolated from proximal colons of DKO adoptive transfer recipients (n = 5 for both groups), converted to cDNA, and analyzed for expression of CD3ε and Foxp3 by real-time RT-PCR. Mean relative expression (expression relative to DKO animal without T-cell transfer) is shown (error bars are 1 SEM). For comparison, values from one unmanipulated WT animal are also shown.
Figure 9
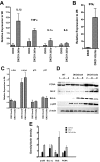
Relative mRNA and protein expression in colonic tissue from _Helicobacter_-infected Smad3/Rag2 DKO mice 1 week after infection. RNA was isolated from colonic epithelial cell preparations or whole colonic tissue, and 2 μg were used to generate cDNA for real-time PCR. Data were normalized to GAPDH expression in each sample. mRNA levels in WT, DKO-broth, and _H. bilis_-infected DKO mice at 1 week after infection are expressed relative to levels in uninfected WT mice. Numbers represent the mean ± SEM, n = 3. There was markedly increased expression of IL-1β and ΤNF-α (13- and 9-fold, respectively) (A), and IFN-γ (42-fold) (B) in whole colonic tissue of infected DKO mice relative to WT mice. ΤNF-α was more moderately increased in uninfected DKO animals relative to uninfected WT-broth mice. C: Levels of c-Myc were constitutively increased two- to threefold in DKO mice and primarily independent of bacterial infection, whereas c-fos, p21, and p15 were not appreciably affected in both uninfected and infected DKO mice. D: For Western analysis, total protein was isolated from epithelial cell preparations or whole colonic tissue, and 30 μg of each sample were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and incubated with appropriate antibodies as described in detail in the text. β-Actin was used as a loading control. For each gel, three samples were included from each group (uninfected WT, DKO-broth, and _H. bilis_-infected DKO). Analysis blotting of proteins from colonic epithelial cells demonstrated increased levels of PCNA and Bcl-2 in _Helicobacter_-infected DKO cells relative to WT and uninfected DKO mice. Bcl-xL was also increased in both uninfected and infected DKO mice. Activation of NF-κB, as indicated by p65 phosphorylation, was noted in uninfected DKO mice, which increased further after Helicobacter infection. E: Plot of densitometry scans of data in D. Densities for each sample were divided by corresponding values for β-actin and average values computed for each group of three. Mean ± SEM is shown. There were significant differences noted between WT and _H. bilis_-infected DKO mice for p65 (P < 0.01), BcL-xL (P < 0.01), _Bcl_II (P < 0.05), and PCNA (P < 0.025). When comparing uninfected DKO to _H. bilis_-infected DKO mice, we noted similar significant differences for all proteins except BcL-xL (ns, P = 0.283).
Similar articles
- Helicobacter infection is required for inflammation and colon cancer in SMAD3-deficient mice.
Maggio-Price L, Treuting P, Zeng W, Tsang M, Bielefeldt-Ohmann H, Iritani BM. Maggio-Price L, et al. Cancer Res. 2006 Jan 15;66(2):828-38. doi: 10.1158/0008-5472.CAN-05-2448. Cancer Res. 2006. PMID: 16424015 Free PMC article. - Characterization of dextran sodium sulfate-induced inflammation and colonic tumorigenesis in Smad3(-/-) mice with dysregulated TGFβ.
Seamons A, Treuting PM, Brabb T, Maggio-Price L. Seamons A, et al. PLoS One. 2013 Nov 11;8(11):e79182. doi: 10.1371/journal.pone.0079182. eCollection 2013. PLoS One. 2013. PMID: 24244446 Free PMC article. - CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice.
Erdman SE, Poutahidis T, Tomczak M, Rogers AB, Cormier K, Plank B, Horwitz BH, Fox JG. Erdman SE, et al. Am J Pathol. 2003 Feb;162(2):691-702. doi: 10.1016/S0002-9440(10)63863-1. Am J Pathol. 2003. PMID: 12547727 Free PMC article. - Nitric oxide and TNF-alpha trigger colonic inflammation and carcinogenesis in Helicobacter hepaticus-infected, Rag2-deficient mice.
Erdman SE, Rao VP, Poutahidis T, Rogers AB, Taylor CL, Jackson EA, Ge Z, Lee CW, Schauer DB, Wogan GN, Tannenbaum SR, Fox JG. Erdman SE, et al. Proc Natl Acad Sci U S A. 2009 Jan 27;106(4):1027-32. doi: 10.1073/pnas.0812347106. Epub 2009 Jan 21. Proc Natl Acad Sci U S A. 2009. PMID: 19164562 Free PMC article. - A tale of two proteins: differential roles and regulation of Smad2 and Smad3 in TGF-beta signaling.
Brown KA, Pietenpol JA, Moses HL. Brown KA, et al. J Cell Biochem. 2007 May 1;101(1):9-33. doi: 10.1002/jcb.21255. J Cell Biochem. 2007. PMID: 17340614 Review.
Cited by
- Persistent infection of rhesus monkeys with 'Helicobacter macacae' and its isolation from an animal with intestinal adenocarcinoma.
Marini RP, Muthupalani S, Shen Z, Buckley EM, Alvarado C, Taylor NS, Dewhirst FE, Whary MT, Patterson MM, Fox JG. Marini RP, et al. J Med Microbiol. 2010 Aug;59(Pt 8):961-969. doi: 10.1099/jmm.0.019117-0. Epub 2010 Apr 22. J Med Microbiol. 2010. PMID: 20413623 Free PMC article. - A bacterial driver-passenger model for colorectal cancer: beyond the usual suspects.
Tjalsma H, Boleij A, Marchesi JR, Dutilh BE. Tjalsma H, et al. Nat Rev Microbiol. 2012 Jun 25;10(8):575-82. doi: 10.1038/nrmicro2819. Nat Rev Microbiol. 2012. PMID: 22728587 Review. - Helicobacter bilis Infection Alters Mucosal Bacteria and Modulates Colitis Development in Defined Microbiota Mice.
Atherly T, Mosher C, Wang C, Hostetter J, Proctor A, Brand MW, Phillips GJ, Wannemuehler M, Jergens AE. Atherly T, et al. Inflamm Bowel Dis. 2016 Nov;22(11):2571-2581. doi: 10.1097/MIB.0000000000000944. Inflamm Bowel Dis. 2016. PMID: 27755267 Free PMC article. - Microbiota disbiosis is associated with colorectal cancer.
Gao Z, Guo B, Gao R, Zhu Q, Qin H. Gao Z, et al. Front Microbiol. 2015 Feb 2;6:20. doi: 10.3389/fmicb.2015.00020. eCollection 2015. Front Microbiol. 2015. PMID: 25699023 Free PMC article. - P58(IPK): a novel "CIHD" member of the host innate defense response against pathogenic virus infection.
Goodman AG, Fornek JL, Medigeshi GR, Perrone LA, Peng X, Dyer MD, Proll SC, Knoblaugh SE, Carter VS, Korth MJ, Nelson JA, Tumpey TM, Katze MG. Goodman AG, et al. PLoS Pathog. 2009 May;5(5):e1000438. doi: 10.1371/journal.ppat.1000438. Epub 2009 May 22. PLoS Pathog. 2009. PMID: 19461876 Free PMC article.
References
- Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405–406. - PubMed
- Clevers H. At the crossroads of inflammation and cancer. Cell. 2004;118:671–674. - PubMed
- Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. J Intern Med. 2000;248:171–183. - PubMed
- Lax AJ, Thomas W. How bacteria could cause cancer: one step at a time. Trends Microbiol. 2002;10:293–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K26 RR024462/RR/NCRR NIH HHS/United States
- 1R01AI053568/AI/NIAID NIH HHS/United States
- R01 AI053568/AI/NIAID NIH HHS/United States
- R01 CA068328/CA/NCI NIH HHS/United States
- 2R01CA68328/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials