Metal A and metal B sites of nuclear RNA polymerases Pol IV and Pol V are required for siRNA-dependent DNA methylation and gene silencing - PubMed (original) (raw)
Metal A and metal B sites of nuclear RNA polymerases Pol IV and Pol V are required for siRNA-dependent DNA methylation and gene silencing
Jeremy R Haag et al. PLoS One. 2009.
Abstract
Plants are unique among eukaryotes in having five multi-subunit nuclear RNA polymerases: the ubiquitous RNA polymerases I, II and III plus two plant-specific activities, nuclear RNA polymerases IV and V (previously known as Polymerases IVa and IVb). Pol IV and Pol V are not required for viability but play non-redundant roles in small interfering RNA (siRNA)-mediated pathways, including a pathway that silences retrotransposons and endogenous repeats via siRNA-directed DNA methylation. RNA polymerase activity has not been demonstrated for Polymerases IV or V in vitro, making it unclear whether they are catalytically active enzymes. Their largest and second-largest subunit sequences have diverged considerably from Pol I, II and III in the vicinity of the catalytic center, yet retain the invariant Metal A and Metal B amino acid motifs that bind magnesium ions essential for RNA polymerization. By using site-directed mutagenesis in conjunction with in vivo functional assays, we show that the Metal A and Metal B motifs of Polymerases IV and V are essential for siRNA production, siRNA-directed DNA methylation, retrotransposon silencing, and the punctate nuclear localization patterns typical of both polymerases. Collectively, these data show that the minimal core sequences of polymerase active sites, the Metal A and B sites, are essential for Pol IV and Pol V biological functions, implying that both are catalytically active.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. Catalytic residues that comprise the Metal A and Metal B binding sites of DNA-dependent RNA polymerases are conserved in the NRPD1, NRPE1/NRPD1b and NRPD2 subunits.
A) Model for the RNA-directed DNA methylation pathway in Arabidopsis. B and C) Positions of NRPD1, NRPE1 and NRPD2 divergence at sites that are invariant in canonical RNA polymerases. The image shows the yeast Pol II Rpb1 and Rbp2 subunits (gray) in complex with the dsDNA substrate (black) and RNA product (red) within Protein Data Bank crystal structure 1R9T (Kornberg laboratory). Amino acids that are invariant among the Arabidopsis Pol I, II and III subunits and yeast Rpb1 or Rpb2, but that are different in NRPD1, NRPE1 or NRPD2, are displayed as spheres. Red spheres highlight the positions of the invariant Metal A and Metal B sites in the largest and second-largest subunits, respectively. Substituted amino acids in the cleft, bridge helix, and active site domains of the largest subunit are colored green, blue and yellow, respectively. Substituted amino acids in the hybrid binding domain of the second-largest subunit are colored magenta. Substituted amino acids in the largest and second-largest subunits that are located outside of these domains are colored cyan. For a complete listing of the highlighted amino acids refer to Table S2. D and E) Multiple protein sequence alignments of RNA polymerase largest and second-largest subunit active site regions. Amino acids highlighted in red and designated by arrows represent the invariant Metal A and Metal B sites. Identical amino acids are highlighted in green and similar amino acids are highlighted in yellow.
Figure 2. Pol IV and Pol V active site amino acids are required for rescue of small RNA production but not Pol IV or Pol V subunit assembly.
A) Acidic amino acids of the Metal A and Metal B sites were mutated to alanines by site-directed mutagenesis. Resulting full-length genomic transgenes were transformed into Arabidopsis nrpd1a-3, nrpd1b-11 (nrpe1) and nrpd2a/2b (nrpd2) homozygous mutants, respectively, as were wild-type versions of each genomic construct. B) RNA blot analysis of small RNAs purified from Arabidopsis inflorescence. Membranes were sequentially probed with body-labeled RNA probes specific for AtCopia, 45S rRNA gene intergenic spacer, 5S rRNA gene intergenic spacer, miR171 or AtSN1 small RNAs. Images of ethidium-bromide stained gels are displayed below the relevant autoradiograms to show that equal amounts of RNA were loaded in each lane. Migration of the 20-nt and 30-nt RNA markers is indicated at the left of each autoradiogram. C and D) Pol IV and Pol V largest subunits bearing active site mutations are indistinguishable from wild-type versions of the proteins in terms of expression level or ability to assemble with the NRPD2 subunit. FLAG-tagged recombinant proteins immunoprecipitated from total protein extracts using anti-FLAG antibodies were detected on immunoblots using FLAG M2 antibody. Membranes were then stripped and re-probed using a polyclonal antibody specific for NRPD2.
Figure 3. Pol IV and Pol V active site amino acids are required for the RNA-directed methylation of 5S rRNA gene repeats.
Southern blot comparison of _Hae_III or _Hpa_II-digested genomic DNA of wild-type (WT), nrpd1a, nrpe1/nrpd1b, and nrpd2 mutants or of transgenic lines generated by transforming these mutants with NRPD1, NRPE1/NRPD1b or NRPD2a full-length transgenes whose sequences are either wild-type or are mutated at the Metal A or Metal B sites. Both wild-type and mutant recombinant proteins have FLAG epitope tags at their carboxyl termini.
Figure 4. DNA methylation and transcriptional silencing of AtSN1 retrotransposons requires the Pol IV and Pol V active sites.
A) Schematic of an AtSN1 retroelement locus showing the locations of _Hae_III restriction enzyme sites and flanking PCR primers. B) AtSN1 DNA methylation analysis using the chop-PCR assay. AtSN1 loci were PCR amplified from _Hae_III digested or undigested genomic DNA and samples were then subjected to agarose gel electrophoresis and staining with ethidium bromide. Locus At2g19920 lacks _Hae_III restriction sites and was used as a control. C) RT-PCR analysis of retrotransposon transcription. Random-primed cDNA was used as the template for PCR amplification of AtSN1 and solo-LTR transcripts. Reactions were then subjected to agarose gel electrophoresis and staining with ethidium bromide. For each genotype, reactions from which reverse transcriptase was omitted (-RT) or for which actin RNA was PCR-amplified serve as controls.
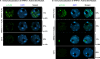
Figure 5. NRPD1 and NRPE1/NRPD1b proteins mutated at their active sites fail to display characteristic Pol IV and Pol V punctate localization patterns in Arabidopsis nuclei.
FLAG epitope-tagged NRPD1 and NRPD1DDD-AAA (panel A) or NRPE1 and NRPE1DDD-AAA (panel B) recombinant proteins were immunolocalized (green signal) using anti-FLAG M2 antibody. Nuclei were counterstained with DAPI (blue signal). The percentage of nuclei showing a given localization pattern and the number of nuclei (n) analyzed are indicated to the right of each panel.
Similar articles
- NRPD4, a protein related to the RPB4 subunit of RNA polymerase II, is a component of RNA polymerases IV and V and is required for RNA-directed DNA methylation.
He XJ, Hsu YF, Pontes O, Zhu J, Lu J, Bressan RA, Pikaard C, Wang CS, Zhu JK. He XJ, et al. Genes Dev. 2009 Feb 1;23(3):318-30. doi: 10.1101/gad.1765209. Genes Dev. 2009. PMID: 19204117 Free PMC article. - Subunit compositions of the RNA-silencing enzymes Pol IV and Pol V reveal their origins as specialized forms of RNA polymerase II.
Ream TS, Haag JR, Wierzbicki AT, Nicora CD, Norbeck AD, Zhu JK, Hagen G, Guilfoyle TJ, Pasa-Tolić L, Pikaard CS. Ream TS, et al. Mol Cell. 2009 Jan 30;33(2):192-203. doi: 10.1016/j.molcel.2008.12.015. Epub 2008 Dec 24. Mol Cell. 2009. PMID: 19110459 Free PMC article. - Functional consequences of subunit diversity in RNA polymerases II and V.
Tan EH, Blevins T, Ream TS, Pikaard CS. Tan EH, et al. Cell Rep. 2012 Mar 29;1(3):208-14. doi: 10.1016/j.celrep.2012.01.004. Cell Rep. 2012. PMID: 22550619 Free PMC article. - Roles of RNA polymerase IV in gene silencing.
Pikaard CS, Haag JR, Ream T, Wierzbicki AT. Pikaard CS, et al. Trends Plant Sci. 2008 Jul;13(7):390-7. doi: 10.1016/j.tplants.2008.04.008. Trends Plant Sci. 2008. PMID: 18514566 Free PMC article. Review. - Multisubunit RNA polymerases IV and V: purveyors of non-coding RNA for plant gene silencing.
Haag JR, Pikaard CS. Haag JR, et al. Nat Rev Mol Cell Biol. 2011 Jul 22;12(8):483-92. doi: 10.1038/nrm3152. Nat Rev Mol Cell Biol. 2011. PMID: 21779025 Review.
Cited by
- Charophytic Green Algae Encode Ancestral Polymerase IV/Polymerase V Subunits and a CLSY/DRD1 Homolog.
Chakraborty T, Trujillo JT, Kendall T, Mosher RA. Chakraborty T, et al. Genome Biol Evol. 2024 Jun 4;16(6):evae119. doi: 10.1093/gbe/evae119. Genome Biol Evol. 2024. PMID: 38874416 Free PMC article. - A conserved Pol II elongator SPT6L mediates Pol V transcription to regulate RNA-directed DNA methylation in Arabidopsis.
Liu Y, Shu J, Zhang Z, Ding N, Liu J, Liu J, Cui Y, Wang C, Chen C. Liu Y, et al. Nat Commun. 2024 May 25;15(1):4460. doi: 10.1038/s41467-024-48940-8. Nat Commun. 2024. PMID: 38796517 Free PMC article. - Structure and mechanism of the plant RNA polymerase V.
Xie G, Du X, Hu H, Li S, Cao X, Jacobsen SE, Du J. Xie G, et al. Science. 2023 Mar 24;379(6638):1209-1213. doi: 10.1126/science.adf8231. Epub 2023 Mar 9. Science. 2023. PMID: 36893216 Free PMC article. - Insights into the molecular mechanisms of CRISPR/Cas9-mediated gene targeting at multiple loci in Arabidopsis.
Zhang Z, Zeng W, Zhang W, Li J, Kong D, Zhang L, Wang R, Peng F, Kong Z, Ke Y, Zhang H, Kim C, Zhang H, Botella JR, Zhu JK, Miki D. Zhang Z, et al. Plant Physiol. 2022 Nov 28;190(4):2203-2216. doi: 10.1093/plphys/kiac431. Plant Physiol. 2022. PMID: 36106983 Free PMC article. - Ovule siRNAs methylate protein-coding genes in trans.
Burgess D, Chow HT, Grover JW, Freeling M, Mosher RA. Burgess D, et al. Plant Cell. 2022 Sep 27;34(10):3647-3664. doi: 10.1093/plcell/koac197. Plant Cell. 2022. PMID: 35781738 Free PMC article.
References
- Cramer P. Multisubunit RNA polymerases. Curr Opin Struct Biol. 2002;12:89–97. - PubMed
- The-Arabidopsis-Genome-Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796–815. - PubMed
- Luo J, Hall BD. A multistep process gave rise to RNA polymerase IV of land plants. J Mol Evol. 2007;64:101–112. - PubMed
- Onodera Y, Haag JR, Ream T, Nunes PC, Pontes O, et al. Plant nuclear RNA polymerase IV mediates siRNA and DNA methylation-dependent heterochromatin formation. Cell. 2005;120:613–622. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources