Regulation of autophagy by the p300 acetyltransferase - PubMed (original) (raw)
Regulation of autophagy by the p300 acetyltransferase
In Hye Lee et al. J Biol Chem. 2009.
Abstract
Autophagy is a regulated process of intracellular catabolism required for normal cellular maintenance, as well as serving as an adaptive response under various stress conditions, including starvation. The molecular regulation of autophagy in mammalian cells remains incompletely understood. Here we demonstrate a role for protein acetylation in the execution and regulation of autophagy. In particular, we demonstrate that the p300 acetyltransferase can regulate the acetylation of various known components of the autophagy machinery. Knockdown of p300 reduces acetylation of Atg5, Atg7, Atg8, and Atg12, although overexpressed p300 increases the acetylation of these same proteins. Furthermore, p300 and Atg7 colocalize within cells, and the two proteins physically interact. The interaction between p300 and Atg7 is dependent on nutrient availability. Finally, we demonstrate that knockdown of p300 can stimulate autophagy, whereas overexpression of p300 inhibits starvation-induced autophagy. These results demonstrate a role for protein acetylation and particularly p300 in the regulation of autophagy under conditions of limited nutrient availability.
Figures
FIGURE 1.
Knockdown of protein acetyltransferases reduces acetylation of endogenous Atg7. A, siRNA-mediated knockdown of p300 results in a reduction of endogenous Atg7 acetylation. Protein acetylation was determined by immunoprecipitation (IP) with an antibody recognizing internal acetyl-lysine residues followed by Western blotting (WB) for Atg7. Immunoprecipitation was performed with 2 mg of protein lysate from HeLa cells transfected with either a control (-) or p300-specific RNAi. Evaluation of the protein input (40 μg) revealed that the change observed in Atg7 acetylation was not accompanied by changes in the level of Atg7 protein expression. B, similar analysis for RNAi-mediated knockdown of CBP. C, RNAi-mediated knockdown of PCAF revealed no obvious change in Atg7 acetylation. Shown is one representative example of three similar experiments.
FIGURE 2.
RNAi-mediated knockdown of p300 reduces acetylation of various Atg constructs. HeLa cells were assessed after control or p300-specific knockdown for the level of Atg acetylation. Various Myc-tagged Atg constructs were employed including the following: A, Atg5; B, Atg7; C, Atg8; and D, Atg12. Level of acetylation was determined by immunoprecipitation (IP) of transfected protein cell lysate (2 mg) with an acetyl-lysine antibody followed by Western blotting (WB) employing the Myc epitope. In each case, p300 knockdown reduced the level of Atg acetylation without altering the level of Atg expression.
FIGURE 3.
Increased expression of p300 augments acetylation of various essential autophagy components. HeLa cells were transiently transfected with the indicated construct encoding for a Myc-tagged Atg family member along with either a p300 expression vector or the empty vector alone (-). Levels of Atg5 (A), Atg7 (B), Atg8 (C), or Atg12 (D) acetylation were determined by immunoprecipitation (IP) of protein lysate (2 mg) using an antibody recognizing internal acetyl-lysine residues followed by Western blotting (WB) employing the Myc epitope. Expression levels of the various transfected myc-Atg constructs were assessed by Western blotting of the protein input (40 μg).
FIGURE 4.
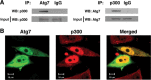
Interaction of Atg7 and p300. A, physical interaction between endogenous p300 and Atg7 is demonstrated by coimmunoprecipitation. HeLa cell protein lysates (4 mg) were immunoprecipitated (IP) with an Atg7-specific antibody or a nonspecific IgG isotype-matched control antibody, and the level of coimmunoprecipitated p300 was determined. The reciprocal immunoprecipitation employing a p300-specific antibody or IgG control is also demonstrated. Levels of p300 and Atg7 in the input lysate (40 μg) are also shown. WB, Western blot. B, subcellular distribution in HeLa cells of transfected Atg7 (green), p300 (red), and their overlap (yellow) are shown.
FIGURE 5.
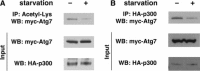
The interaction between p300 and Atg7 is regulated by external nutrients. A, levels of Atg7 acetylation fall under starved conditions. Atg7 acetylation was assessed under fed (-) conditions or following 2 h of starvation (+). Although total levels of Atg7 were unchanged, overall acetylation was reduced following nutrient withdrawal. B, interaction between p300 and Atg7 is reduced under starved conditions. Protein-protein interaction was determined by immunoprecipitation (IP) of epitope-tagged p300 and assessment of coimmunoprecipitated Atg7. Although levels of both p300 and Atg7 were unchanged under fed or starved conditions, the interaction between p300 and Atg7 was routinely reduced ∼40% (fed, 1.0 ± 0.2; starved, 0.6 ± 0.1; n = 3 separate experiments; p < 0.05). WB, Western blot.
FIGURE 6.
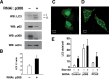
Knockdown of p300 augments autophagy. HeLa cells were assessed after either control or p300-specific RNAi knockdown. A, induction of autophagy under fed conditions was monitored by the conversion of LC3-I to LC3-II, whereas steady state rates of autophagy were analyzed by Western blot (WB) analysis for p62 expression. Levels of p300 expression and actin (loading control) are also shown. B, quantification of LC3-II to LC3-I ratio in control versus the p300-specific knockdown cells. Shown are the results (mean ± S.D.) of three separate experiments (*, p < 0.05). C, representative confocal image of the localization of GFP-LC3 in cells transfected with a control siRNA, or D, in cells transfected with an siRNA directed against p300. E, quantification of “LC3 dots” using transient transfection of a construct encoding a GFP-LC3 chimeric protein along with the indicated RNAi. Knockdown of p300 appears to stimulate autophagosome formation under both fed and starved conditions. In contrast, knockdown of PCAF, which appeared to have little effect on Atg acetylation, also had little to no effect on autophagy.
FIGURE 7.
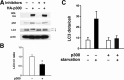
Increased expression of p300 inhibits autophagy. HeLa cells were assessed following transfection with either an empty vector (-) or a vector encoding the p300 acetyltransferase. A, induction of autophagy was assessed by analyzing the conversion of LC3–1 to LC3-II under starved conditions. Steady state autophagy was monitored by assessing the level of p62 expression. Expression of the epitope-tagged p300 and actin (loading control) is also demonstrated. Protein levels were assessed either in the absence or presence of lysosomal protease inhibitors. WB, Western blot. B, autophagy induction as assessed by the ratio of LC3-II to LC3-I is demonstrated following p300 expression. Shown are the results (mean ± S.D.) of three separate experiments performed in the absence of lysosomal protease inhibitors (*, p < 0.05). C, expression of p300 suppresses starvation-induced autophagy as assessed by the GFP-LC3 dot assay.
Similar articles
- Interaction of caveolin-1 with ATG12-ATG5 system suppresses autophagy in lung epithelial cells.
Chen ZH, Cao JF, Zhou JS, Liu H, Che LQ, Mizumura K, Li W, Choi AM, Shen HH. Chen ZH, et al. Am J Physiol Lung Cell Mol Physiol. 2014 Jun 1;306(11):L1016-25. doi: 10.1152/ajplung.00268.2013. Epub 2014 Apr 11. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24727585 Free PMC article. - The FAP motif within human ATG7, an autophagy-related E1-like enzyme, is essential for the E2-substrate reaction of LC3 lipidation.
Tanida I, Yamasaki M, Komatsu M, Ueno T. Tanida I, et al. Autophagy. 2012 Jan;8(1):88-97. doi: 10.4161/auto.8.1.18339. Epub 2012 Jan 1. Autophagy. 2012. PMID: 22170151 - BAT3 modulates p300-dependent acetylation of p53 and autophagy-related protein 7 (ATG7) during autophagy.
Sebti S, Prébois C, Pérez-Gracia E, Bauvy C, Desmots F, Pirot N, Gongora C, Bach AS, Hubberstey AV, Palissot V, Berchem G, Codogno P, Linares LK, Liaudet-Coopman E, Pattingre S. Sebti S, et al. Proc Natl Acad Sci U S A. 2014 Mar 18;111(11):4115-20. doi: 10.1073/pnas.1313618111. Epub 2014 Mar 3. Proc Natl Acad Sci U S A. 2014. PMID: 24591579 Free PMC article. - Molecules and their functions in autophagy.
Pyo JO, Nah J, Jung YK. Pyo JO, et al. Exp Mol Med. 2012 Feb 29;44(2):73-80. doi: 10.3858/emm.2012.44.2.029. Exp Mol Med. 2012. PMID: 22257882 Free PMC article. Review. - ATG12-ATG3 connects basal autophagy and late endosome function.
Murrow L, Debnath J. Murrow L, et al. Autophagy. 2015;11(6):961-2. doi: 10.1080/15548627.2015.1040976. Autophagy. 2015. PMID: 25998418 Free PMC article. Review.
Cited by
- How to control self-digestion: transcriptional, post-transcriptional, and post-translational regulation of autophagy.
Feng Y, Yao Z, Klionsky DJ. Feng Y, et al. Trends Cell Biol. 2015 Jun;25(6):354-63. doi: 10.1016/j.tcb.2015.02.002. Epub 2015 Mar 8. Trends Cell Biol. 2015. PMID: 25759175 Free PMC article. Review. - Histone acetyltransferase MoHat1 acetylates autophagy-related proteins MoAtg3 and MoAtg9 to orchestrate functional appressorium formation and pathogenicity in Magnaporthe oryzae.
Yin Z, Chen C, Yang J, Feng W, Liu X, Zuo R, Wang J, Yang L, Zhong K, Gao C, Zhang H, Zheng X, Wang P, Zhang Z. Yin Z, et al. Autophagy. 2019 Jul;15(7):1234-1257. doi: 10.1080/15548627.2019.1580104. Epub 2019 Feb 18. Autophagy. 2019. PMID: 30776962 Free PMC article. - The crosstalk of NAD, ROS and autophagy in cellular health and ageing.
Sedlackova L, Korolchuk VI. Sedlackova L, et al. Biogerontology. 2020 Jun;21(3):381-397. doi: 10.1007/s10522-020-09864-0. Epub 2020 Mar 3. Biogerontology. 2020. PMID: 32124104 Free PMC article. Review. - Melatonin regulates aging and neurodegeneration through energy metabolism, epigenetics, autophagy and circadian rhythm pathways.
Jenwitheesuk A, Nopparat C, Mukda S, Wongchitrat P, Govitrapong P. Jenwitheesuk A, et al. Int J Mol Sci. 2014 Sep 22;15(9):16848-84. doi: 10.3390/ijms150916848. Int J Mol Sci. 2014. PMID: 25247581 Free PMC article. Review. - Epigenetic Control of Autophagy Related Genes Transcription in Pulpitis via JMJD3.
Yin B, Ma Q, Zhao L, Song C, Wang C, Yu F, Shi Y, Ye L. Yin B, et al. Front Cell Dev Biol. 2021 Aug 9;9:654958. doi: 10.3389/fcell.2021.654958. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34434926 Free PMC article.
References
- Ohsumi, Y. (2001) Nat. Rev. Mol. Cell Biol. 2, 211-216 - PubMed
- Kuma, A., Hatano, M., Matsui, M., Yamamoto, A., Nakaya, H., Yoshimori, T., Ohsumi, Y., Tokuhisa, T., and Mizushima, N. (2004) Nature 432, 1032-1036 - PubMed
- Sarbassov, D. D., Ali, S. M., and Sabatini, D. M. (2005) Curr. Opin. Cell Biol. 17, 596-603 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous