Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials - PubMed (original) (raw)
. 2009 Feb 20;27(6):872-7.
doi: 10.1200/JCO.2008.19.5362. Epub 2009 Jan 5.
Alberto Sobrero, Axel Grothey, Michael J O'Connell, Marc Buyse, Thierry Andre, Yan Zheng, Erin Green, Roberto Labianca, Chris O'Callaghan, Jean Francois Seitz, Guido Francini, Daniel Haller, Greg Yothers, Richard Goldberg, Aimery de Gramont
Affiliations
- PMID: 19124803
- PMCID: PMC2738431
- DOI: 10.1200/JCO.2008.19.5362
Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials
Daniel Sargent et al. J Clin Oncol. 2009.
Abstract
Purpose: Limited data are available on the time course of treatment failures (recurrence and/or death), the nature and duration of adjuvant treatment benefit, and long-term recurrence rates in patients with resected stage II and III colon cancer.
Methods: The data set assembled by the Adjuvant Colon Cancer Endpoints Group, a collection of individual patient data from 18 trials and more than 20,800 patients testing fluorouracil-based adjuvant therapy in patients with stage II or III colon cancer, was analyzed.
Results: A significant overall survival (OS) benefit of adjuvant therapy was consistent over the 8-year follow-up period. The risk of recurrence in patients treated with adjuvant chemotherapy never exceeds that of control patients, signifying that adjuvant therapy cures some patients, as opposed to delaying recurrence. After 5 years, recurrence rates were less than 1.5% per year, and after 8 years, they were less than 0.5% per year. Significant disease-free survival (DFS) benefit from adjuvant chemotherapy was observed in the first 2 years. After 2 years, DFS rates in treated and control patients were not significantly different, and after 4 years, no trend toward benefit was demonstrated. This benefit was primarily driven by patients with stage III disease.
Conclusion: Adjuvant chemotherapy provides significant DFS benefit, primarily by reducing the recurrence rate, within the first 2 years of adjuvant therapy with some benefit in years 3 to 4, translating into long-term OS benefit. This reflects the curative role of chemotherapy in the adjuvant setting. After 5 years, recurrence rates in patients treated on clinical trials are low, and after 8 years, they are minimal; thus, long-term follow-up for recurrence is of little value.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
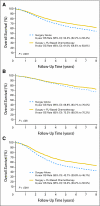
Fig 1.
Kaplan-Meier plots of overall survival (OS) in treatment arm versus control arm for (A) all patients, (B) stage II patients, and (C) stage III patients. FU, fluorouracil.
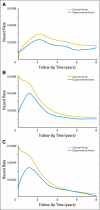
Fig 2.
Hazard rates by time from random assignment and treatment arm. Plots of the hazard rate for (A) overall survival, (B) disease-free survival, and (C) time to recurrence by arm (treatment and control) over the 8-year follow-up period. The y axis plots the risk of recurrence on a daily scale; thus, for example, the value 0.0002 represents an annualized 7.3% risk of an event (0.0002 × 365 = 0.073).
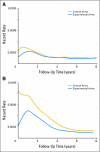
Fig 3.
Disease-free survival hazard rates by time from random assignment and treatment arm in (A) stage II patients and (B) stage III patients.
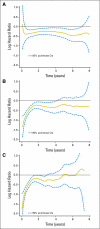
Fig 4.
Continuous time estimate of the log-hazard ratios for (A) overall survival, (B) disease-free survival, and (C) time to recurrence comparing treatment arm with control arm over the 8-year follow-up period, with 95% pointwise CIs. Values less than 0 indicate a benefit from treatment. For example, a value of −0.50 for the log-hazard ratio indicates a hazard ratio of 0.61 comparing treatment with control at that time point (e–0.50 = 0.61), that is, a 39% reduction in the risk of an event.
Fig 5.
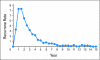
Risk of recurrence in each 6-month interval after random assignment among patients remaining recurrence free at the start of each interval, by time.
Similar articles
- Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials.
Sargent DJ, Wieand HS, Haller DG, Gray R, Benedetti JK, Buyse M, Labianca R, Seitz JF, O'Callaghan CJ, Francini G, Grothey A, O'Connell M, Catalano PJ, Blanke CD, Kerr D, Green E, Wolmark N, Andre T, Goldberg RM, De Gramont A. Sargent DJ, et al. J Clin Oncol. 2005 Dec 1;23(34):8664-70. doi: 10.1200/JCO.2005.01.6071. Epub 2005 Oct 31. J Clin Oncol. 2005. PMID: 16260700 - Association between disease-free survival and overall survival when survival is prolonged after recurrence in patients receiving cytotoxic adjuvant therapy for colon cancer: simulations based on the 20,800 patient ACCENT data set.
de Gramont A, Hubbard J, Shi Q, O'Connell MJ, Buyse M, Benedetti J, Bot B, O'Callaghan C, Yothers G, Goldberg RM, Blanke CD, Benson A, Deng Q, Alberts SR, Andre T, Wolmark N, Grothey A, Sargent D. de Gramont A, et al. J Clin Oncol. 2010 Jan 20;28(3):460-5. doi: 10.1200/JCO.2009.23.1407. Epub 2009 Dec 14. J Clin Oncol. 2010. PMID: 20008641 Free PMC article. - Benefits and adverse events in younger versus older patients receiving adjuvant chemotherapy for colon cancer: findings from the Adjuvant Colon Cancer Endpoints data set.
Hubbard J, Thomas DM, Yothers G, Green E, Blanke C, O'Connell MJ, Labianca R, Shi Q, Bleyer A, de Gramont A, Sargent D. Hubbard J, et al. J Clin Oncol. 2012 Jul 1;30(19):2334-9. doi: 10.1200/JCO.2011.41.1975. Epub 2012 May 21. J Clin Oncol. 2012. PMID: 22614981 Free PMC article. - Adjuvant therapy for colon cancer in the new millenium.
Rao S, Cunningham D. Rao S, et al. Scand J Surg. 2003;92(1):57-64. doi: 10.1177/145749690309200109. Scand J Surg. 2003. PMID: 12705552 Review. - Association Between Adjuvant Chemotherapy Duration and Survival Among Patients With Stage II and III Colon Cancer: A Systematic Review and Meta-analysis.
Boyne DJ, Cuthbert CA, O'Sullivan DE, Sajobi TT, Hilsden RJ, Friedenreich CM, Cheung WY, Brenner DR. Boyne DJ, et al. JAMA Netw Open. 2019 May 3;2(5):e194154. doi: 10.1001/jamanetworkopen.2019.4154. JAMA Netw Open. 2019. PMID: 31099875 Free PMC article.
Cited by
- Colorectal Cancer Recurrence Prediction Using a Tissue-Free Epigenomic Minimal Residual Disease Assay.
Nakamura Y, Tsukada Y, Matsuhashi N, Murano T, Shiozawa M, Takahashi Y, Oki E, Goto M, Kagawa Y, Kanazawa A, Ohta T, Ouchi A, Bando H, Uchigata H, Notake C, Ikematsu H, Yoshino T. Nakamura Y, et al. Clin Cancer Res. 2024 Oct 1;30(19):4377-4387. doi: 10.1158/1078-0432.CCR-24-1651. Clin Cancer Res. 2024. PMID: 39110016 Free PMC article. - Capecitabine as Maintenance Therapy for High-Risk, Resected Colorectal Cancer.
Auber ML, Wen S, Hobbs G, Higa GM. Auber ML, et al. Gastrointest Tumors. 2021 Apr;8(2):81-86. doi: 10.1159/000513960. Epub 2021 Mar 10. Gastrointest Tumors. 2021. PMID: 33981686 Free PMC article. - Continuous sensing of IFNα by hepatic endothelial cells shapes a vascular antimetastatic barrier.
Tran NL, Ferreira LM, Alvarez-Moya B, Buttiglione V, Ferrini B, Zordan P, Monestiroli A, Fagioli C, Bezzecchi E, Scotti GM, Esposito A, Leone R, Gnasso C, Brendolan A, Guidotti LG, Sitia G. Tran NL, et al. Elife. 2022 Oct 25;11:e80690. doi: 10.7554/eLife.80690. Elife. 2022. PMID: 36281643 Free PMC article. - Estimating Population-Based Recurrence Rates of Colorectal Cancer over Time in the United States.
Kunst N, Alarid-Escudero F, Aas E, Coupé VMH, Schrag D, Kuntz KM. Kunst N, et al. Cancer Epidemiol Biomarkers Prev. 2020 Dec;29(12):2710-2718. doi: 10.1158/1055-9965.EPI-20-0490. Epub 2020 Sep 30. Cancer Epidemiol Biomarkers Prev. 2020. PMID: 32998946 Free PMC article. - A subset of genetic susceptibility variants for colorectal cancer also has prognostic value.
Noci S, Dugo M, Bertola F, Melotti F, Vannelli A, Dragani TA, Galvan A. Noci S, et al. Pharmacogenomics J. 2016 Apr;16(2):173-9. doi: 10.1038/tpj.2015.35. Epub 2015 May 12. Pharmacogenomics J. 2016. PMID: 25963333
References
- Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990;322:352–358. - PubMed
- Wolmark N, Rockette H, Mamounas EP, et al. Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes' B and C carcinoma of the colon: Results from National Surgical Adjuvant Breast and Bowel Project C-04. J Clin Oncol. 1999;17:3553–3559. - PubMed
- Wolmark N, Rockette H, Fisher B, et al. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: Results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. J Clin Oncol. 1993;11:1879–1887. - PubMed
- O'Connell MJ, Laurie JA, Kahn M, et al. Prospectively randomized trial of postoperative adjuvant chemotherapy in patients with high-risk colon cancer. J Clin Oncol. 1998;16:295–300. - PubMed
- André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, 5-fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA 37377/CA/NCI NIH HHS/United States
- U10 CA012027/CA/NCI NIH HHS/United States
- CA 69651/CA/NCI NIH HHS/United States
- CA 12027/CA/NCI NIH HHS/United States
- U10 CA069974/CA/NCI NIH HHS/United States
- U10 CA025224/CA/NCI NIH HHS/United States
- CA 69974/CA/NCI NIH HHS/United States
- U10 CA037377/CA/NCI NIH HHS/United States
- CA 25224/CA/NCI NIH HHS/United States
- U10 CA069651/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources