Mammalian nicotinic acetylcholine receptors: from structure to function - PubMed (original) (raw)
Review
Mammalian nicotinic acetylcholine receptors: from structure to function
Edson X Albuquerque et al. Physiol Rev. 2009 Jan.
Abstract
The classical studies of nicotine by Langley at the turn of the 20th century introduced the concept of a "receptive substance," from which the idea of a "receptor" came to light. Subsequent studies aided by the Torpedo electric organ, a rich source of muscle-type nicotinic receptors (nAChRs), and the discovery of alpha-bungarotoxin, a snake toxin that binds pseudo-irreversibly to the muscle nAChR, resulted in the muscle nAChR being the best characterized ligand-gated ion channel hitherto. With the advancement of functional and genetic studies in the late 1980s, the existence of nAChRs in the mammalian brain was confirmed and the realization that the numerous nAChR subtypes contribute to the psychoactive properties of nicotine and other drugs of abuse and to the neuropathology of various diseases, including Alzheimer's, Parkinson's, and schizophrenia, has since emerged. This review provides a comprehensive overview of these findings and the more recent revelations of the impact that the rich diversity in function and expression of this receptor family has on neuronal and nonneuronal cells throughout the body. Despite these numerous developments, our understanding of the contributions of specific neuronal nAChR subtypes to the many facets of physiology throughout the body remains in its infancy.
Figures
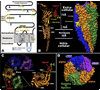
FIG. 1
Basic structure of nicotinic acetylcholine receptors (nAChRs). A: the basic linear sequence of all nAChR subunits appears as a large extracellular domain, four transmembrane domains, and a cytoplasmic domain of variable size that resides between TM3 and TM4. This produces the classic “3+1” designation that describes this structure. Also characterizing the superfamily of receptor to which nAChRs belong is the Cys-loop that is composed of two disulfide-linked cystines separated by 13 amino acids that are highly conserved. Subunits that have the Cys-Cys pair are designated as α subunits (see text). Amino acids conserved in most nAChRs are identified using the Torpedo α subunit numbering system (476). Residues in green are important to the α subunit contribution to the agonist-binding pocket and orange residues are important to the β or negative face of the agonist-binding site. Orange residues with black dots are required for gating the channel. Amino acids in TM2 important to establishing the channel gate are in gray, and those important to relieving the gate are in blue. Residues lining the pore (green) are important to determine ion selectivity and conductance such as E241 that in part determines the permeability to Ca2+. The lone cysteine418 in TM4 contributes to measuring the response of nAChRs dependent on the lipid environment. The blue “Y” are _N_-linked glycosylation sites whose relative locations (except near the Cys-loop) vary among subunits. B: the EM structure of the Torpedo nAChR is from Unwin (476), and images were generated using the UCSF-chimera program with coordinates obtained from the Protein Data Bank ID 1OED.pdb. The approximate dimensions of the intact Torpedo receptor are given. An α subunit is shown where ribbons designate the secondary structures of the primary sequence. The extracellular domain is largely β-sheets and all TMs are α-helices. Note that the TM domains are believed to extend ~ 10 Å beyond the membrane. The cytoplasmic domain is depicted as a large α-helix, although this is likely to vary in size and complexity of structure between subunits (see text). This is an α subunit as designated by the C-loop harboring the Cys-Cys pair that projects from the extracellular domain core-β structure to surround an agonist ligand. The Cys-loop position near the extracellular end is noted. The entire receptor complex with a solid surface is shown to the right. Note the cone shape of the receptor and that the subunits are tilted relative to the 90° plane of the membrane. Also, the projection of the C-loop towards the adjacent subunit in the counterclockwise direction is apparent. C: looking down on the receptor from the extracellular side reveals the arrangement of 5 subunits around the central pore, which is lined by the TM2 from each subunit. Note that the agonist-binding site is contained in a pocket between the α and adjacent non-α subunit defined on its outer face by the Cys-Cys pair. One α subunit is removed from the complex and ribbons are added to the structure to designate secondary structure as in B. The arrangement of β strands in a barrel-like portion directly over the TM domains is seen. Also, the extension of the C-loop around ligand is evident. D: similar to B, the receptor surface is added to show the relative positioning of each subunit (as labeled) and to look directly down the pore. The extracellular domains form the mouth of the pore, which is strongly constricted by a residue in TM2 that forms the gate and reduces the diameter of the non-ligand bound receptor to ~3 Å.
FIG. 2
The ligand binding site and the proposed mechanism for gating the ion pore. A: in this depiction, an agonist-binding α subunit (dark blue) and a structural β subunit (in light blue) are shown with a solid surface looking from the extracellular side with the subunit pair slightly tipped away from the pore. When agonist is bound (as shown for nicotine, red), the α C-loop is moved towards the structural subunit to cap the agonist-binding site and effectively encase the ligand in the deep cleft formed between the subunits. The α Cys-Cys pair (–188) is in yellow. Other residues interacting with the ligand from the α subunit are colored green and from the β subunit are colored in orange. The circled region is enlarged and the surface removed to reveal in B the amino acids within the agonist-binding site that interact with nicotine. The same color scheme is used, and the residues interacting to form the agonist-binding site are named and numbered. The arrows indicate β-strand structure. The weak lines interacting with nicotine (whose electrostatic surface is in light red) are hydrogen bonds. Certain key residues include tryptophan 143 (W143) from the α subunit which contributes to forming the base of the agonist-binding site and α-tyrosine 185 (Y185), which is important to stabilize the ligand within the pocket upon entry. In the α5 nAChR subunit, this residue is an aspartic acid that introduces a potentially negatively charged group into the pocket to inhibit ligand binding. As indicated by the extent of the molecular surface of nicotine (shown in transparent red), these hydrophobic residues from both subunit faces further stabilize the ligand in the pocket through van der Waals interactions, and other residues not shown (including D85, located near W143) also contribute to ligand binding through stabilizing the position of pocket residues. [Adapted from the 2.7-Å resolution X-ray structure of the AChBP (Protein Data Bank ID 1I9B.pdb) and the images generated in UCSF Chimera by Pettersen et al. (375).] B: upon binding of agonist and capping of the ligand-binding site (1), rotational motion in the β-strands is transmitted through the subunit (2) to residues that are near the TM domain-membrane interface. At this point, the rotational motion imparts two important interactions. The first is to move the loop between β-strands β1 and β2 towards the linking sequence of TM2 and TM3. This positions an invariant valine (V44) into the hydrophobic pocket that is created by the proximity of proline-272 (P272) and serine-269 (S269). These amino acids, or conservative changes, are present in most nAChRs. At the same time, the β10 strand moves counterclockwise to position arginine-209 (R209) towards glutamic acid-45 (E45; also β1 strand) to form an ionic (salt) bond. These interactions result in the rotation of TM4 ~15° to move the hydrophobic gating residues [valines (V255) and (V259) and leucine (L251)] away from the pore and the polar S248 and S252 toward the widened channel. The relief of the gate allows the channel to completely hydrate and conduct ions (5). Residues at the extracellular and intracellular faces (e.g., E241) ring the channel. These residues vary among subunits and receptors as polar and/or charged and contribute to determining the relative ion current through the pore. Also, highly charged rings of amino acids such as E241 enhance certain ion permeability such as by Ca2+. [Model shown is based on the original study of Unwin (475) taken from electron microscopy studies of channel gating from the Torpedo nAChR (Protein Data Bank code 2BG9) and from high-resolution studies of the AChBPs (see text for details).]
FIG. 3
Toxins that have coevolved to interact with nAChRs can be agonists or antagonists. A strong force in driving evolutionary success is the interrelationship between predator-prey strategies. Because the origin of nAChR dates to the earliest of organisms, and these receptors have acquired important roles in animal motility and nervous system function, they are excellent targets both for predation and defense. Shown above are structural models of binding between nAChRs and a variety of toxins. Toxins are in red, the α subunit is in dark blue, and the structural β subunit is in light blue. For α-cobratoxin, the protein surface was added to show the very tight fit between the toxin and the nAChR binding site. Several points are made. 1) The toxins come in a variety of forms. This includes the elaborate proteins produced in snake venoms to the simple molecules of plants used for defense against predation. 2) The toxins can function as either agonists or antagonists. 3) Note the interaction between toxin and receptor that is in general centered at the ligand binding site (note the yellow Cys-Cys pair that usually wraps the toxin at the site of ligand interaction). The exquisite refinement of toxin structure to bind the nAChR also indicates that this site in the nAChR has remained relatively invariant through its evolutionary history.
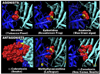
FIG. 4
Nicotinic receptor modulation of hippocampal inhibitory circuitry. A: choline (10 mM) induces type IA currents in hippocampal interneurons of different species of animals and in human cortical interneurons. Type IA current results from activation of α7 nAChRs because it is sensitive to blockade by nanomolar concentrations of methyllycaconitine (MLA) or α-bungarotoxin (α-BGT). Type IA current is not blocked by bupropion (1 µM) or nicotine (100 nM), but is partially inhibited by DHβE (10 µM) or choline (50 µM). Nicotinic responses with these characteristics are not detected in neurons of mice with a null mutation in the gene that encodes the α7 nAChR subunit. In the presence of MLA (10 nM), ACh but not choline induces type II current in the interneurons. The poor efficacy of cytisine to induce type II current and the blockade of this current by DHβE (10 µM) indicate that it results from activation of α4β2 nAChRs. A low degree of activation of these nAChRs by ACh (10 µM) fails to induce action potentials; however, it triggers GABAergic PSCs in the interneurons, suggesting that the nAChRs are located on preterminal regions. Type II current is not sensitive to blockade by α-BGT (100 nM) or choline (100 µM) but is partially inhibited by bupropion (1 µM) or nicotine (100 nM). In the presence of MLA (10 nM), nicotinic agonists induce type III responses (AMPA EPSCs at −68 mV and NMDA EPSCs at +40 mV); the order of agonist efficacy is cytisine > ACh > choline. At 1 µM, mecamylamine inhibits type III nAChR responses. Furthermore, type III responses are blocked by nicotine (100 nM), bupropion (1 µM), or choline (30 µM). The pharmacological profile of type III responses suggests that they result from activation of α3β4/β2 nAChRs. B: choline-induced type IA current results in action potentials in interneurons and IPSCs in pyramidal neurons. As expected, MLA and tetrodotoxin blocked both types of events. C: concentration-response relationships for choline- and nicotine-induced inhibition of type IA, II, and III responses recorded from CA1 SR interneurons in rat hippocampal slices (16). D: a diagram of the major neurons in the CA1 field of the hippocampus and how the different nAChR subtypes modulate various aspects of inhibitory circuitry. In the pyramidal layer (py) there are the excitatory pyramidal neurons that are glutamatergic (GLU; green) and pyramidal associated interneurons that are GABAergic (GABA; dark blue). These interneurons extend dendrites both in the direction of the stratum radiatum (SR) where they interact with Schaffer collaterals (Schaf. Col.) and terminate in the stratum lacunosum moleculare (SLM) to interact with perforant path fibers. The majority of nAChRs on these neurons are of the type I (α7) subtype, which can also be located on some principal excitatory neurons. Axons, which also express type II (α4β2) nAChRs, extend from interneurons to interact with many excitatory neurons and other interneurons. In some cases, they can extend to other hippocampal fields via the alveus (alv). Other inhibitory interneurons expressing nAChRs (light blue) are located in the SR and stratum oriens (SO). The SR interneurons often express nAChRs of the types I and II. Type III (α3β4β2) nAChRs are present on glutamate axons innervating SR interneurons and possibly other interneurons. To the right, immunolocalization of nAChR expression in a coronal section of the mouse hippocampus CA1 that is matched approximately to the diagram is also shown. Colored arrows identify examples of the interneurons diagrammed in their respective region.
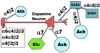
FIG. 5
A diagram of nAChR control of dopamine neurotransmission of the basal ganglia system. This diagram shows the complex regulation of dopamine release by excitatory (Glu), inhibitory (GABA), and cholinergic (ACh) neurons. A complex variety of nAChRs participate in regulating these circuits as indicated by their differential subunit composition and location on neurons of different types. While subunit composition is indicated, this is not strictly defined and additional subtypes, especially those incorporating α5, are likely to participate in modulating this circuit. [Adapted from Gotti and Clementi (184) and Wonnacott (502).]
FIG. 6
Schematic representation of intracellular signaling resulting from activation of nAChRs, glutamate ionotropic receptors, and GABAA receptors. In the CA1 field of the hippocampus, a single interneuron can express somatodendritic α7 and α4β2 nAChRs and receive α3β4/β2 nAChR-regulated glutamatergic inputs. Thus there is the potential that intracellular signaling is regulated by the cross-talk of the various transmitter systems. During a low degree of activation of α7 nAChRs and α3β4β2 nAChRs, for instance, Ca2+ may enter the cells through nAChRs or NMDA receptors and favor phosphorylation of the transcription factor CREB, which in turn modifies gene expression (82). If there is intense stimulation of all three nAChRs, the resulting depolarization can trigger activation of VGCC, which in turn would activate the calcineurin pathway and prevent CREB activation. A concurrent activation of preterminal α4β2 nAChRs would hyperpolarize the neuron via GABAergic inhibition and prevent activation of the VGCC. Such a sequential interplay between nicotinic and GABAergic signaling has been shown to guide neuronal development in the hippocampus and other regions (281).
FIG. 7
Regulation of nAChRs by nontraditional ligands. In this illustration, an agonist-binding α subunit (light blue) and a structural β subunit (dark blue) are shown with a solid surface looking from the extracellular side with either nicotine alone (left) or nicotine and galantamine (right). Photoaffinity labeling studies carried out using [3H]physostigmine and mapping of the epitope of the monoclonal antibody FK1 revealed that the region flanking the amino acid Lys-125 on the nAChR α subunit contains essential elements of the physostigmine-binding site and is highly conserved among different α subunits and across species (372, 413, 415). The galantamine-binding region is close to, but distinct from, the classical agonist-binding region. The galantamine-binding region is highly hydrophobic. As described in the text, elements that appear essential for binding of galantamine to the AChBP include the tryptophan residues 147 and 149, the tyrosine residue 93 or 55, and to a lesser extent the tyrosine residue 195. It has also been proposed that the dipole between the carbonyl group of the tryptophan residues and the protonated nitrogen of galantamine may be strengthened by the anionic side chain of the residue aspartate 89 (207). The crystallographic study of the AChBP-galantamine complex also revealed that some of the residues that contact galantamine in the complex are conserved among non-α nAChR subunits, suggesting that galantamine may bind to both α- and non-α interfaces (207).
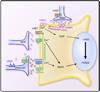
FIG. 8
Role of astrocyte-derived kynurenic acid (KYNA) in regulating the activity of dopaminergic neurons in the ventral tegmental area. This simplified scheme illustrates the role of astrocyte-derived KYNA in modulating synaptic transmission between a cortical glutamatergic axon and a dopaminergic neuron in the ventral tegmental area (VTA). The VTA supplies dopaminergic inputs to several nuclei in the so-called reward circuit. This circuit, which is centered around the nucleus accumbens and is critical for animals to display goal-directed behaviors, has been shown to be strategically positioned to relay information about motivation, drive, and affective state to motor systems. Dopaminergic activity in the nucleus accumbens has an essential role in the functioning of this circuit. The reinforcing properties of drugs of abuse, including nicotine, are associated with increased dopaminergic activity in the nucleus accumbens, which receives dopaminergic inputs from the VTA. The nucleus accumbens receives input from several limbic structures, including the amygdala, the hippocampus, and the medial prefrontal cortex and innervates the ventral pallidum, subpallidal area, and substantia nigra, which provide inputs to motor structures. The dopaminergic activity in the nucleus accumbens is, thus, regulated by local integration of various neurotransmitter systems originating in different areas of the brain. However, it is also controlled by the cortical glutamatergic input to the dopaminergic neurons in the VTA. Local infusion in the rat striatum of a kynurenine hydroxylase inhibitor has been shown to increase extracellular levels of dopamine, which were decreased by the addition of KYNA to the perfusate (390, 391). The association between low levels of KYNA and increased levels of dopamine has also been observed in mice with a null mutation in the gene that encodes the KATII enzyme (516). Thus it is tempting to speculate that dopaminergic transmission in the striatum is regulated by astrocyte-derived KYNA. VTA dopaminergic neurons are known to express somatodendritic α7 nAChRs and to receive excitatory inputs from cortical glutamatergic terminals that express α7 nAChRs. Activation of these receptors stimulates dopamine release into the nucleus accumbens within the striatum. It is plausible to hypothesize that endogenous KYNA released from astrocytes may inhibit tonically active α7 nAChRs and, thereby, decrease dopamine levels in the striatum. This emphasizes the concept of tripartite synapses in the brain, whereby synaptic activity is tuned by astrocyte-derived regulatory signals (480).
Similar articles
- Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system.
Dani JA, Bertrand D. Dani JA, et al. Annu Rev Pharmacol Toxicol. 2007;47:699-729. doi: 10.1146/annurev.pharmtox.47.120505.105214. Annu Rev Pharmacol Toxicol. 2007. PMID: 17009926 Review. - Neuronal nicotinic acetylcholine receptor expression and function on nonneuronal cells.
Gahring LC, Rogers SW. Gahring LC, et al. AAPS J. 2006 Jan 13;7(4):E885-94. doi: 10.1208/aapsj070486. AAPS J. 2006. PMID: 16594641 Free PMC article. Review. - Nicotinic acetylcholine receptors: from structure to brain function.
Hogg RC, Raggenbass M, Bertrand D. Hogg RC, et al. Rev Physiol Biochem Pharmacol. 2003;147:1-46. doi: 10.1007/s10254-003-0005-1. Epub 2003 Mar 20. Rev Physiol Biochem Pharmacol. 2003. PMID: 12783266 Review. - Neuronal Nicotinic Acetylcholine Receptor Structure and Function and Response to Nicotine.
Dani JA. Dani JA. Int Rev Neurobiol. 2015;124:3-19. doi: 10.1016/bs.irn.2015.07.001. Epub 2015 Aug 21. Int Rev Neurobiol. 2015. PMID: 26472524 Free PMC article. Review. - The role of neuronal nicotinic acetylcholine receptors in acute and chronic neurodegeneration.
O'Neill MJ, Murray TK, Lakics V, Visanji NP, Duty S. O'Neill MJ, et al. Curr Drug Targets CNS Neurol Disord. 2002 Aug;1(4):399-411. doi: 10.2174/1568007023339166. Curr Drug Targets CNS Neurol Disord. 2002. PMID: 12769612 Review.
Cited by
- Characterization of a novel α-conotoxin from conus textile that selectively targets α6/α3β2β3 nicotinic acetylcholine receptors.
Luo S, Zhangsun D, Wu Y, Zhu X, Hu Y, McIntyre M, Christensen S, Akcan M, Craik DJ, McIntosh JM. Luo S, et al. J Biol Chem. 2013 Jan 11;288(2):894-902. doi: 10.1074/jbc.M112.427898. Epub 2012 Nov 26. J Biol Chem. 2013. PMID: 23184959 Free PMC article. - Nicotinic Receptor Subunits Atlas in the Adult Human Lung.
Diabasana Z, Perotin JM, Belgacemi R, Ancel J, Mulette P, Delepine G, Gosset P, Maskos U, Polette M, Deslée G, Dormoy V. Diabasana Z, et al. Int J Mol Sci. 2020 Oct 9;21(20):7446. doi: 10.3390/ijms21207446. Int J Mol Sci. 2020. PMID: 33050277 Free PMC article. - Cholinergic Functioning, Cognition, and Anticholinergic Medication Burden in Schizophrenia.
Joshi YB. Joshi YB. Curr Top Behav Neurosci. 2023;63:393-406. doi: 10.1007/7854_2022_400. Curr Top Behav Neurosci. 2023. PMID: 36441495 Review. - Activation of α7 nicotinic acetylcholine receptors increases intracellular cAMP levels via activation of AC1 in hippocampal neurons.
Cheng Q, Yakel JL. Cheng Q, et al. Neuropharmacology. 2015 Aug;95:405-14. doi: 10.1016/j.neuropharm.2015.04.016. Epub 2015 Apr 29. Neuropharmacology. 2015. PMID: 25937212 Free PMC article. - A Genetic Animal Model of Alcoholism for Screening Medications to Treat Addiction.
Bell RL, Hauser S, Rodd ZA, Liang T, Sari Y, McClintick J, Rahman S, Engleman EA. Bell RL, et al. Int Rev Neurobiol. 2016;126:179-261. doi: 10.1016/bs.irn.2016.02.017. Epub 2016 Mar 21. Int Rev Neurobiol. 2016. PMID: 27055615 Free PMC article. Review.
References
- Adcock C, Smith GR, Sansom MS. The nicotinic acetylcholine receptor: from molecular model to single-channel conductance. Eur Biophys J. 2000;29:29–37. - PubMed
- Aisen PS. Evaluation of selective COX-2 inhibitors for the treatment of Alzheimer’s disease. J Pain Symptom Manage. 2002;23:S35–S40. - PubMed
- Akinshola BE, Taylor RE, Ogunseitan AB, Onaivi ES. Anandamide inhibition of recombinant AMPA receptor subunits in Xenopus oocytes is increased by forskolin and 8-bromo-cyclic AMP. Naunyn-Schmiedebergs Arch Pharmacol. 1999;360:242–248. - PubMed
- Albuquerque EX, Alkondon M, Pereira EFR, Castro NG, Schrattenholz A, Barbosa CT, Bonfante-Cabarcas R, Aracava Y, Eisenberg HM, Maelicke A. Properties of neuronal nicotinic acetylcholine receptors: pharmacological characterization and modulation of synaptic function. J Pharmacol Exp Ther. 1997;280:1117–1136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 NS059344/NS/NINDS NIH HHS/United States
- U01 NS059344-02/NS/NINDS NIH HHS/United States
- P01 HL072903/HL/NHLBI NIH HHS/United States
- R01 NS025296-17/NS/NINDS NIH HHS/United States
- U01 NS059344-01/NS/NINDS NIH HHS/United States
- P01-HL-72903/HL/NHLBI NIH HHS/United States
- R01 NS025296/NS/NINDS NIH HHS/United States
- R01 NS025296-19/NS/NINDS NIH HHS/United States
- U01-NS-59344/NS/NINDS NIH HHS/United States
- AG-17517/AG/NIA NIH HHS/United States
- NS-25296/NS/NINDS NIH HHS/United States
- U01 NS059344-03/NS/NINDS NIH HHS/United States
- R01 AG017517/AG/NIA NIH HHS/United States
- R01 NS025296-18/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases