AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633 - PubMed (original) (raw)
AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633
Zhen Chen et al. Circ Res. 2009.
Abstract
Endothelial nitric oxide synthase (eNOS) plays a central role in maintaining cardiovascular homeostasis by controlling NO bioavailability. The activity of eNOS in vascular endothelial cells (ECs) largely depends on posttranslational modifications, including phosphorylation. Because the activity of AMP-activated protein kinase (AMPK) in ECs can be increased by multiple cardiovascular events, we studied the phosphorylation of eNOS Ser633 by AMPK and examined its functional relevance in the mouse models. Shear stress, atorvastatin, and adiponectin all increased AMPK Thr172 and eNOS Ser633 phosphorylations, which were abolished if AMPK was pharmacologically inhibited or genetically ablated. The constitutively active form of AMPK or an AMPK agonist caused a sustained Ser633 phosphorylation. Expression of gain-/loss-of-function eNOS mutants revealed that Ser633 phosphorylation is important for NO production. The aorta of AMPKalpha2(-/-) mice showed attenuated atorvastatin-induced eNOS phosphorylation. Nano-liquid chromatography/tandem mass spectrometry (LC/MS/MS) confirmed that eNOS Ser633 was able to compete with Ser1177 or acetyl-coenzyme A carboxylase Ser79 for AMPKalpha phosphorylation. Nano-LC/MS/MS confirmed that eNOS purified from AICAR-treated ECs was phosphorylated at both Ser633 and Ser1177. Our results indicate that AMPK phosphorylation of eNOS Ser633 is a functional signaling event for NO bioavailability in ECs.
Figures
Figure 1
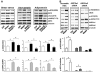
Shear stress, statin, and adiponectin enhance phosphorylation of AMPK Thr172 and eNOS Ser635 in BAECs. Confluent monolayers of BAECs were preexposed to laminar shear stress at 5 dyn/cm2 for 6 hours, which was increased to 12 dyn/cm2 for the durations shown (A), treated with atorvastatin at 1 _μ_mol/L (B), or adiponectin at 30 μ_g/mL (C) for the indicated times. The cells were then lysed and underwent SDS-PAGE, followed by Western blotting with various primary antibodies. The bar graphs below are densitometry quantifications of the ratios of phospho-eNOS at Ser635 or Ser1179 to total eNOS and that of phospho-AMPK Thr172 to total AMPK_α. The data are means±SD from 3 independent experiments, with static cells (A) or untreated controls (B and C) set as 1. *P<0.05 compared to control groups.
Figure 2
AMPK phosphorylates eNOS Ser635 in cultured ECs. Confluent BAECs were treated with various concentrations of AICAR for 15 minutes (A) or infected with Ad-AMPK-CA at different multiplicities of infection (MOI) for 24 hours (B). The control cells were infected with Ad-null virus at 50 multiplicities of infection. Cell lysates were analyzed by Western blotting with the indicated antibodies. C, BAECs were treated with AICAR at 1 mmol/L for 15 minutes or left untreated as controls. AMPK_α_ was immunoprecipitated from cell lysates with the use of anti–pan-AMPK_α_ at 1:20 or 1:15 dilution. Rabbit IgG was used as an IP control. The kinase activity of immunoprecipitated AMPK_α_ was assayed with recombinant GST-eNOS (wild type [WT]), GST-eNOS (S1179A), or GST-eNOS (S635A) as substrates. Left, The expressed GST-eNOS is shown by Coomassie blue staining and Western blotting. The phosphorylation of GST-eNOS S1179 and S635 by the immunoprecipitated AMPK_α_ was detected by Western blotting. The level of immunoprecipitated AMPK_α_ and GST-eNOS used in the assays was also shown by immunoblotting. Data represent results from 3 independent experiments.
Figure 3
AMPK inhibition attenuates eNOS Ser635 phosphorylation in ECs. A, BAECs were pretreated with compound C at 20 _μ_mol/L for 30 minutes before undergoing 30-minute treatment with laminar shear stress at 12 dyn/cm2, atorvastatin at 1 _μ_mol/L, or adiponectin at 30 _μ_g/mL. In B, HUVECs were transfected with AMPK_α_1 siRNA, AMPK_α_2 siRNA, or scramble RNA (10 nmol/L) before atorvastatin treatment. Phosphorylation of eNOS Ser635, AMPK Thr172, and ACC Ser79 was revealed by Western blotting. The bar graphs represent the densitometry analyses of Western blotting as means±SD of 3 or 4 independent experiments. *P<0.05 between groups as indicated.
Figure 4
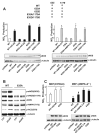
AMPK mediates eNOS Ser633 phosphorylation in mouse aortas in vivo. C57BL6 (A) or AMPKα2−/− (B) mice were given atorvastatin at 50 mg/kg body weight for the indicated times before euthanasia. In the control group, mice received the same volume (0.5 mL) of saline 6 hours before euthanasia. Tissue extracts from 2 aortas were pooled into 1 sample to be analyzed by Western blotting with various antibodies as indicated. The bar graphs are results of densitometry analyses of the ratio of phospho-eNOS to total eNOS, phospho-AMPK, and phosphor-ACC to α-tubulin. The saline controls were set as 1. Data represent means±SD from 3 independent experiments. *P<0.05 between atorvastatin-treated and control mice.
Figure 5
AMPK is necessary for eNOS S635-mediated NO bioavailability. A, HEK293 cells were transfected with various plasmids expressing the wild-type (WT) and mutated eNOS (ie, 635A, 635D, 635A/1179A, and 635D/1179D). One set of cells transfected with WT or 635A was treated with AICAR (1 mmol/L), and in parallel experiments, another set was infected with Ad-AMPKα2-CA (100 multiplicities of infection). The NO bioavailability from various cells was determined by Griess assay and expressed as NOx. In all experiments, the NOx produced from cells transfected with pcDNA3 was considered background and thus subtracted from total NOx values of all cell groups. On Western blotting, the relative level of expressed eNOS was normalized to that of α-tubulin. NOx production was further normalized to the relative level of eNOS. B, HEK293 cells transfected with WT or 635A eNOS were treated with AICAR (1 mmol/L) for 15 minutes. Cell lysates were resolved on SDS-PAGE and subjected to Western blotting with various antibodies as indicated. C, MEFs isolated from C57BL6 or AMPKα2−/− mice were transfected with various eNOS plasmids in the presence or absence of AICAR or coinfected with Ad-AMPK-CA, as indicated. NOx production was measured accordingly. In A, NOx produced from HEK293 cells transfected with WT-eNOS was set as 1. In C, NOx value corresponding to C57BL6 MEFs transfected with WT-eNOS was set as 1. Data are means±SD from 5 independent experiments. In A, *P<0.05 compared with HEK293 cells transfected with WT eNOS. In C, *P<0.05 between C57BL6 MEFs and AMPKα2−/− MEFs transfected with WT-eNOS; #P<0.05 between C57BL6 MEFs and AMPKα2−/− MEFs transfected with WT-eNOS and then treated with AICAR.
Figure 6
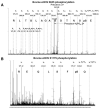
Competition between eNOS Ser633/635 and Ser1177/1179 for AMPK phosphorylation detected by LC/MS. Shown in A are peptide sequences of SAMS and those adjacent to human ACC1 Ser79, human eNOS Ser633, and human eNOS Ser1177. The sequences shown indicate the synthesized S633 and S1177 oligopeptides. B, BAECs were treated with AICAR (1 mmol/L) for 15 minutes and lysed. AMPK was immunoprecipitated from BAEC lysates by anti-pan-AMPKα. SAMS, S633, or S1177 (1 mmol/L) together with (γ-32P) ATP (8 μCi) were mixed with the immunoprecipitated AMPKα for IP kinase activity assays. The phosphorylation of SAMS, S633, and S1177 peptides was determined by the incorporation of 32P. The scintillation counts of various samples were normalized to that of control containing reaction cocktail (40 μL), SAMS (10 μL), and lysis buffer (50 μL) set as 1. *P<0.05 compared with control. C, SAMS and S1177 peptides were mixed at ratios of 1:0, 1:1, and 1:10, and the peptide mixture was included in AMPKα IP kinase assays. Nano-LC/MS was performed to detect the phosphorylated SAMS and S1177. The spectra show m/z around 466 and 802, with the dashed, solid, and dotted lines representing SAMS: S1177 at 1:0, 1:1, and 1:10, respectively. Phosphorylated SAMS and eNOS633 peptides were detected as positive ions with m/z 465.49, 4+, and m/z 571.79, 4+, respectively. Quantitation of signal intensity for individual ions was based on the maximal apex-peak height (ie, ion counts) displayed on the m/z spectrum derived from summing all individual scans across the entire retention time of the corresponding ion on the extracted ion chromatogram. The baseline background was subtracted from the above peak height to obtain extracted ion total counts (EITC), which was then used to quantify the changes of phosphorylation level for each peptide, with the highest value set as 1. Data are means±SD from triplicate experiments. Similar analyses were performed to assess the competition between SAMS and S633 (D) or that between S633 and S1177 (E) for AMPKα phosphorylation.
Figure 7
LC/MS/MS analysis of eNOS Ser635 and Ser1179 phosphorylation in BAECs. eNOS immunoprecipitated from AICAR-treated BAECs was trypsin-digested and then passed through TiO2- coated magnetic beads to enrich phosphopeptides. Nano-LC/MS/MS was used to map the phosphorylation site within the peptides containing Ser635 (A) or Ser1179 (B).
Comment in
- When metabolism rules perfusion: AMPK-mediated endothelial nitric oxide synthase activation.
Schulz E, Schuhmacher S, Münzel T. Schulz E, et al. Circ Res. 2009 Feb 27;104(4):422-4. doi: 10.1161/CIRCRESAHA.109.194274. Circ Res. 2009. PMID: 19246685 No abstract available.
Similar articles
- AMP-activated protein kinase promotes the differentiation of endothelial progenitor cells.
Li X, Han Y, Pang W, Li C, Xie X, Shyy JY, Zhu Y. Li X, et al. Arterioscler Thromb Vasc Biol. 2008 Oct;28(10):1789-95. doi: 10.1161/ATVBAHA.108.172452. Epub 2008 Jul 3. Arterioscler Thromb Vasc Biol. 2008. PMID: 18599796 Free PMC article. - AMP-activated protein kinase inhibits homocysteine-induced dysfunction and apoptosis in endothelial progenitor cells.
Jia F, Wu C, Chen Z, Lu G. Jia F, et al. Cardiovasc Drugs Ther. 2011 Feb;25(1):21-9. doi: 10.1007/s10557-010-6277-1. Cardiovasc Drugs Ther. 2011. PMID: 21258964 - Adenosine monophosphate activated protein kinase regulates ABCG1-mediated oxysterol efflux from endothelial cells and protects against hypercholesterolemia-induced endothelial dysfunction.
Li D, Zhang Y, Ma J, Ling W, Xia M. Li D, et al. Arterioscler Thromb Vasc Biol. 2010 Jul;30(7):1354-62. doi: 10.1161/ATVBAHA.110.204230. Epub 2010 Apr 15. Arterioscler Thromb Vasc Biol. 2010. PMID: 20395595 - Activation and signaling by the AMP-activated protein kinase in endothelial cells.
Fisslthaler B, Fleming I. Fisslthaler B, et al. Circ Res. 2009 Jul 17;105(2):114-27. doi: 10.1161/CIRCRESAHA.109.201590. Circ Res. 2009. PMID: 19608989 Review. - Advances in the molecular mechanisms of statins in regulating endothelial nitric oxide bioavailability: Interlocking biology between eNOS activity and L-arginine metabolism.
Chen WH, Chen CH, Hsu MC, Chang RW, Wang CH, Lee TS. Chen WH, et al. Biomed Pharmacother. 2024 Feb;171:116192. doi: 10.1016/j.biopha.2024.116192. Epub 2024 Jan 22. Biomed Pharmacother. 2024. PMID: 38262153 Review.
Cited by
- Atorvastatin enhance efficacy of mesenchymal stem cells treatment for swine myocardial infarction via activation of nitric oxide synthase.
Song L, Yang YJ, Dong QT, Qian HY, Gao RL, Qiao SB, Shen R, He ZX, Lu MJ, Zhao SH, Geng YJ, Gersh BJ. Song L, et al. PLoS One. 2013 May 31;8(5):e65702. doi: 10.1371/journal.pone.0065702. Print 2013. PLoS One. 2013. PMID: 23741509 Free PMC article. - Adrenocorticotropic Hormone and PI3K/Akt Inhibition Reduce eNOS Phosphorylation and Increase Cortisol Biosynthesis in Long-Term Hypoxic Ovine Fetal Adrenal Cortical Cells.
Newby EA, Kaushal KM, Myers DA, Ducsay CA. Newby EA, et al. Reprod Sci. 2015 Aug;22(8):932-41. doi: 10.1177/1933719115570899. Epub 2015 Feb 5. Reprod Sci. 2015. PMID: 25656500 Free PMC article. - Autophagy as a Guardian of Vascular Niche Homeostasis.
Dergilev K, Gureenkov A, Parfyonova Y. Dergilev K, et al. Int J Mol Sci. 2024 Sep 20;25(18):10097. doi: 10.3390/ijms251810097. Int J Mol Sci. 2024. PMID: 39337582 Free PMC article. Review. - AMPK: a therapeutic target of heart failure-not only metabolism regulation.
Li X, Liu J, Lu Q, Ren D, Sun X, Rousselle T, Tan Y, Li J. Li X, et al. Biosci Rep. 2019 Jan 3;39(1):BSR20181767. doi: 10.1042/BSR20181767. Print 2019 Jan 31. Biosci Rep. 2019. PMID: 30514824 Free PMC article. Review. - Regulation of cardiac miR-208a, an inducer of obesity, by rapamycin and nebivolol.
Gul R, Mahmood A, Luck C, Lum-Naihe K, Alfadda AA, Speth RC, Pulakat L. Gul R, et al. Obesity (Silver Spring). 2015 Nov;23(11):2251-9. doi: 10.1002/oby.21227. Epub 2015 Sep 18. Obesity (Silver Spring). 2015. PMID: 26381051 Free PMC article.
References
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. - PubMed
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. - PubMed
- Stuehr DJ. Mammalian nitric oxide synthases. Biochim Biophys Acta. 1999;1411:217–230. - PubMed
- Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. - PubMed
- Loscalzo J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ Res. 2001;88:756–762. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL77448/HL/NHLBI NIH HHS/United States
- R01 GM066151-05/GM/NIGMS NIH HHS/United States
- R01 HL089940/HL/NHLBI NIH HHS/United States
- HL89940/HL/NHLBI NIH HHS/United States
- R01 HL077448/HL/NHLBI NIH HHS/United States
- R01 HL077448-04/HL/NHLBI NIH HHS/United States
- R01 GM066151/GM/NIGMS NIH HHS/United States
- R01 HL089940-01A2/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases