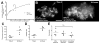The role of the granuloma in expansion and dissemination of early tuberculous infection - PubMed (original) (raw)
The role of the granuloma in expansion and dissemination of early tuberculous infection
J Muse Davis et al. Cell. 2009.
Abstract
Granulomas, organized aggregates of immune cells, form in response to persistent stimuli and are hallmarks of tuberculosis. Tuberculous granulomas have long been considered host-protective structures formed to contain infection. However, work in zebrafish infected with Mycobacterium marinum suggests that granulomas contribute to early bacterial growth. Here we use quantitative intravital microscopy to reveal distinct steps of granuloma formation and assess their consequence for infection. Intracellular mycobacteria use the ESX-1/RD1 virulence locus to induce recruitment of new macrophages to, and their rapid movement within, nascent granulomas. This motility enables multiple arriving macrophages to efficiently find and phagocytose infected macrophages undergoing apoptosis, leading to rapid, iterative expansion of infected macrophages and thereby bacterial numbers. The primary granuloma then seeds secondary granulomas via egress of infected macrophages. Our direct observations provide insight into how pathogenic mycobacteria exploit the granuloma during the innate immune phase for local expansion and systemic dissemination.
Figures
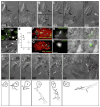
Figure 1. The Early Granuloma Enhances Bacterial Replication by RD1-Dependent Recruitment and Infection of New Cells
(A and B) Fluorescent images of WT (A) and ΔRD1 (B) infected lesions at 48, 72, and 96 hr post-infection (hpi). Scale bars, 10 μm. (C and D) Three-dimensional reconstructions of granulomas in WT (C) and ΔRD1 (D) infection, showing Hoechst-positive nuclei rendered white (indicated by white arrows in D) accumulated over 24 hr. Scale grid, 5 μm per square. (E) Comparison of numbers of Hoechst-positive arrivals in WT and ΔRD1 lesions after 24 hr. Brackets indicate granulomas in the same embryo. Horizontal lines represent means. p value from Mann-Whitney test. (F–H) Neutral red-stained optic tectum macrophages at 5 days post-hindbrain injection with WT Mm(F), ΔRD1 Mm(G), or no infection (H). Macrophages detected using neutral red dye (upper panels) and their outlines overlayed on images of the fluorescent bacteria present (lower panels—no fluorescent image collected for uninfected embryos). Scale bars, 100 μm. (I) Numbers of optic tectum macrophages at 2 dpi and 5 dpi. p = 0.47 (2 dpi) and 0.01 (5 dpi) (Kruskal-Wallis test, p < 0.05).
Figure 2. Uninfected Cells at WT Granulomas Show Distinct Morphology and Rapid Motility and Infection
(A–C) Hoechst-positive nuclei (blue) are distinguishable as uninfected (A) or infected (B). White arrows, Hoechst-positive nuclei; arrowheads, bacteria (green). (C) Infected Hoechst-positive cells in WT versus ΔRD1 lesions over 24 hr. p from Mann-Whitney test. (D and E) Distinct morphologies of uninfected cells at WT (D) and ΔRD1 (E) lesions. (D) Left: highly motile cell at WT granuloma with lamellipodium (white bracket), elongated nucleus (black bracket), and uropod (large black arrowhead). Arrow indicates direction of travel. Right: distinct appearance in WT granulomas of highly vesicular macrophage shortly before phagocytosis. Small black arrowheads, vesicles. Scale bars, 5 μm. (E) Less motile cell at ΔRD1 granuloma with no lamellipodium and rounded nucleus (black bracket). Scale bar, 5 μm. (F) Comparison of overall cell length, left, and nuclear aspect ratio, right, of uninfected cells at WT and ΔRD1 granulomas. p by unpaired Student’s t test. (G) Speeds of uninfected cells at WT or ΔRD1 granulomas, or at the site of injection 1 hr post-infection in the hindbrain ventricle. Bracket above indicates results of 1 way ANOVA (Kruskal-Wallis test)—uninfected cells at ΔRD1 granulomas (***) differed significantly from all others (p < 0.005), other differences not significant. (H–K) Tracks of uninfected cells in (G). (H and I) Cells at WT and ΔRD1 granulomas—all cells tracked for each strain from one granuloma. Insets, fluorescence view of whole granuloma. Inset scale bar, 30 μm. (J and K) Cells at 1 hr post-injection. 1 hpi tracks from two WT-infected embryos and three ΔRD1-infected embryos. All scale bars = 5 μm unless noted otherwise.
Figure 3. Death and Phagocytosis of Infected Macrophages
(A–C) DIC time lapse of death and phagocytosis of infected macrophage. (A) two focal planes at time zero showing bacteria in cell with healthy nucleus (large black arrow), with uninfected cell (small white arrow and brackets as in Figure 2D) passing by. Dashed arrow, direction of travel. (B) Same infected cell as in (A), before (top) and after (bottom) nuclear collapse. (C) Same cell after further nuclear collapse into apoptotic sphere and later time points showing complete phagocytosis by new macrophage (black and white brackets and white arrow). Small black arrowheads, vesicles in approaching macrophage. (D) Hoechst (white) and bacterial GFP (green) overlay above, with matching DIC below, showing compact Hoechst-positive apoptotic nuclei (black arrows) associated with bacteria (white arrowheads), compared to diffuse Hoechst staining of live nuclei (white arrow). (E) Infected Hoechst-positive apoptotic cells per granuloma in WT and ΔRD1 lesions over 24 hr. p from Mann-Whitney test. (F–I) Apoptotic cells in WT granulomas detected by acridine orange (F and H) or annexin V (G and I). (F) Granuloma of red fluorescent Mm with acridine orange-positive cells (white arrows). Bracket indicates area shown in (H). (G) Granuloma of red fluorescent Mm with annexin V-positive cells (white arrows). Bracket indicates area shown in (I). (H) Acridine orange signal (left) corresponds with DIC appearance of apoptotic bodies (dotted circle, middle). Overlay, right. Scale bar, 10 μm. (I) Annexin V signal (left) corresponds with DIC appearance of apoptotic bodies (dotted circle, middle). Overlay, right. Scale bar, 10 μm. (J) DIC time-lapse images of single macrophage (nucleus indicated by white arrow) pulling a small group of bacteria (white arrowheads) from a larger cluster (X). Below, sketches derived from these panels with additional details from other planes at same time point. All scale bars, 5 μm.
Figure 4. Bacterial Expansion in Early Granulomas as a Function of Macrophage Arrival, Infection, Death, and Rephagocytosis
(A) Correlation of bacterial growth in granulomas, measured by fluorescence area with arrival of new macrophages. Data from eight granulomas in seven embryos. Bracket indicates pair of granulomas imaged in the same embryo. (B) Deconvolved fluorescence images of granuloma at time 0 (left) and 24 hr (right). Scale bar, 20 μm. (C and D) Bacterial expansion in five granulomas over 24 hr as measured by (C) fluorescence area and (D) number of infected cells. (E) Fold growth as measured in panels (C) and (D) as compared to predicted fold growth based on mathematical modeling. Differences not significant (ANOVA).
Figure 5. Motility and Departure of Infected Granuloma Macrophages
(A) Average speeds of infected and uninfected cells versus their bacterial volumes. (B) Tracks of departing infected macrophages from Movie S11. Scale bar, 50 μm. (C) Bacterial volumes and average speeds of departing macrophages in (B), compared to those that did not depart. p by Student’s t test. (D–F) Departure of infected macrophages from brain granuloma. (D) Granuloma immediately after photoactivation (red) and (E) 24 hr later, demonstrating granuloma growth and departure of infected macrophages (arrowheads). (F) DIC/red fluorescence overlay of departing macrophages (arrowheads). Dotted line represents granuloma edge. Scale bars in (D)–(F), 20 μm. (G–I) Hematogenous dissemination from tail granuloma. (G) A single granuloma (arrow) photoactivated at 3 dpi. Scale bar, 300 μm. (H) At 4 dpi, photoactivated bacteria (arrowheads) seen in gill vasculature. (I) DIC image of cluster #2 from (H). Dashed line: limits of vasculature, e: erythrocyte, m: muscle. Scale bar, 10 μm.
Figure 6. Departing Bacteria form Secondary Granulomas
(A–C) Bacteria from one granuloma spread to another. (A) Primary granuloma immediately after photoactivation at 3 dpi. Scale bar, 50 μm. (B) At 5 dpi, the original granuloma (1) persists and a new one (2) has appeared nearby. Dashed box, area shown in panel C. (C) Photoactivated bacteria from granuloma 1 spreading in granuloma 2. Scale bar, 10 μm. (D) Single granuloma at 3 dpi (white arrow, 1) with departure of bacteria to new location 24 hr later (white arrowhead). By 48 hr, the original granuloma (1) continues to grow, the new locus is forming a granuloma (2), and a third locus of infection has appeared (arrowhead). Scale bar, 300 μm. (E) Dissemination from single primary granulomas in ten embryos. Horizontal lines indicate fate of departing macrophages (remain single or form granuloma) on successive days. *Indicates dissemination of multiple macrophages that could not be tracked definitively between days or that appeared on the last day of tracking. The embryo from (D) is shown as 1.
Figure 7. Mechanisms and Consequences of Early Granuloma Formation and the Impact of RD1
Top: WT pathogenesis. An infected cell (1) recruits new macrophages and induces their rapid motility (2). Upon its death, it is phagocytosed by the recruited cells (3). After more bacterial growth, these infected cells also die (4) and are phagocytosed by more recruited macrophages (5). Infected cells egress the primary granuloma (6) to initiate secondary granulomas (7). Bottom: The same events altered by the absence of RD1. An infected cell allows intracellular bacterial growth similar to WT. The death of this cell is delayed compared to WT (1), and the recruited macrophages are fewer in number and lack the rapid motility seen in WT (2). Slower infected macrophage death and rephagocytosis of dead cells (3) combine to produce small, delayed granulomas with better containment of infection (4).
Comment in
- Who benefits from granulomas, mycobacteria or host?
Bold TD, Ernst JD. Bold TD, et al. Cell. 2009 Jan 9;136(1):17-9. doi: 10.1016/j.cell.2008.12.032. Cell. 2009. PMID: 19135882 Free PMC article.
Similar articles
- Looking within the zebrafish to understand the tuberculous granuloma.
Ramakrishnan L. Ramakrishnan L. Adv Exp Med Biol. 2013;783:251-66. doi: 10.1007/978-1-4614-6111-1_13. Adv Exp Med Biol. 2013. PMID: 23468113 Review. - Mycobacterium marinum infection of adult zebrafish causes caseating granulomatous tuberculosis and is moderated by adaptive immunity.
Swaim LE, Connolly LE, Volkman HE, Humbert O, Born DE, Ramakrishnan L. Swaim LE, et al. Infect Immun. 2006 Nov;74(11):6108-17. doi: 10.1128/IAI.00887-06. Infect Immun. 2006. PMID: 17057088 Free PMC article. - Tuberculous granuloma formation is enhanced by a mycobacterium virulence determinant.
Volkman HE, Clay H, Beery D, Chang JC, Sherman DR, Ramakrishnan L. Volkman HE, et al. PLoS Biol. 2004 Nov;2(11):e367. doi: 10.1371/journal.pbio.0020367. Epub 2004 Oct 26. PLoS Biol. 2004. PMID: 15510227 Free PMC article. - Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death.
Clay H, Volkman HE, Ramakrishnan L. Clay H, et al. Immunity. 2008 Aug 15;29(2):283-94. doi: 10.1016/j.immuni.2008.06.011. Immunity. 2008. PMID: 18691913 Free PMC article. - New models for the study of Mycobacterium-host interactions.
Pozos TC, Ramakrishnan L. Pozos TC, et al. Curr Opin Immunol. 2004 Aug;16(4):499-505. doi: 10.1016/j.coi.2004.05.011. Curr Opin Immunol. 2004. PMID: 15245746 Review.
Cited by
- Microarray analysis of the in vitro granulomatous response to Mycobacterium tuberculosis H37Ra.
Reyes N, Bettin A, Reyes I, Geliebter J. Reyes N, et al. Colomb Med (Cali). 2015 Mar 30;46(1):26-32. eCollection 2015 Jan-Mar. Colomb Med (Cali). 2015. PMID: 26019382 Free PMC article. - Within the Enemy's Camp: contribution of the granuloma to the dissemination, persistence and transmission of Mycobacterium tuberculosis.
Shaler CR, Horvath CN, Jeyanathan M, Xing Z. Shaler CR, et al. Front Immunol. 2013 Feb 14;4:30. doi: 10.3389/fimmu.2013.00030. eCollection 2013. Front Immunol. 2013. PMID: 23420646 Free PMC article. - Isoniazid resistance without a loss of fitness in Mycobacterium tuberculosis.
Lee JH, Ammerman NC, Nolan S, Geiman DE, Lun S, Guo H, Bishai WR. Lee JH, et al. Nat Commun. 2012 Mar 20;3:753. doi: 10.1038/ncomms1724. Nat Commun. 2012. PMID: 22434196 Free PMC article. - Genome-scale analyses of transcriptional start sites in Mycobacterium marinum under normoxic and hypoxic conditions.
Huang S, Zhou W, Tang W, Zhang Y, Hu Y, Chen S. Huang S, et al. BMC Genomics. 2021 Apr 6;22(1):235. doi: 10.1186/s12864-021-07572-8. BMC Genomics. 2021. PMID: 33823801 Free PMC article.
References
- Algood HM, Chan J, Flynn JL. Chemokines and tuberculosis. Cytokine Growth Factor Rev. 2003;14:467–477. - PubMed
- Andersen P. Host responses and antigens involved in protective immunity to Mycobacterium tuberculosis. Scand J Immunol. 1997;45:115–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI54503/AI/NIAID NIH HHS/United States
- R01 AI036396/AI/NIAID NIH HHS/United States
- R01 AI36396/AI/NIAID NIH HHS/United States
- R37 AI054503/AI/NIAID NIH HHS/United States
- R01 AI054503/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials