The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility - PubMed (original) (raw)
The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility
Xinnan Wang et al. Cell. 2009.
Abstract
Mitochondria are mobile organelles and cells regulate mitochondrial movement in order to meet the changing energy needs of each cellular region. Ca(2+) signaling, which halts both anterograde and retrograde mitochondrial motion, serves as one regulatory input. Anterograde mitochondrial movement is generated by kinesin-1, which interacts with the mitochondrial protein Miro through an adaptor protein, milton. We show that kinesin is present on all axonal mitochondria, including those that are stationary or moving retrograde. We also show that the EF-hand motifs of Miro mediate Ca(2+)-dependent arrest of mitochondria and elucidate the regulatory mechanism. Rather than dissociating kinesin-1 from mitochondria, Ca(2+)-binding permits Miro to interact directly with the motor domain of kinesin-1, preventing motor/microtubule interactions. Thus, kinesin-1 switches from an active state in which it is bound to Miro only via milton, to an inactive state in which direct binding to Miro prevents its interaction with microtubules. Disrupting Ca(2+)-dependent regulation diminishes neuronal resistance to excitotoxicity.
Figures
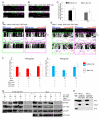
Figure 1. Milton Recruits KHC to Neuronal Mitochondria
(A) Model of the Miro/milton/KHC complex that mediates anterograde transport of mitochondria on microtubules (MT). The EF-hands of Miro are marked in blue. (B) The endogenous motor/adaptor complex from HEK 293T cells was immunoprecipitated with anti-hMiro1 and probed with anti-KHC, anti-hMiro1, and anti-OIP106 (one of two human homologs of milton). Milton, Miro, and KHC were precipitated by anti-hMiro1, but not by a control rabbit IgG. Input lanes were loaded with one fifth the amount of homogenate used for the precipitations. The entire precipitate was loaded in the IP lanes. (C) KHC is localized to mitochondria in the presence (ii) but not the absence (i) of milton. Neurites of rat hippocampal neurons transfected with KHC-mCit (green), Miro-Myc (blue) and RFP-mito (red). KHC-mCit is diffuse in the absence of milton but mitochondrial when milton is coexpressed. Scale bars, 10 μm. (D) The coimmunoprecipitation of Miro-Myc and KHC-ECFP from HEK cells requires milton. Complexes (IP) were precipitated from cell lysates (Input), in Ca++-free buffer, with anti-GFP. Input lanes contained one fifth the amount of homogenate immunoprecipitated, and were probed with anti-tubulin to control for differences in protein inputs.
Figure 2. Ca++ Influx Inhibits Axonal Transport of Hippocampal Mitochondria
(A) A representative axon of a neuron transfected with the axonal marker synaptophysin-YFP (SYP-YFP, green), and RFP-mito (magenta). (B) Application of calcimycin inhibited mitochondrial movements. The first frame of the live-imaging movie (top panel) and kymographs generated from the movie, in which the x axis represents the mitochondrial position and the y axis is time. Vertical white lines represent stationary mitochondria and diagonal lines represent moving mitochondria. Below each kymograph are hand-drawn traces of the diagonal lines, in which anterograde movements are red and retrograde blue. Scale bars, 10 μm.
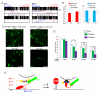
Figure 3. EF-hand Mutations in Miro Prevent the Inhibition by Ca++ of Mitochondrial Axonal Transport
(A,B) Mitochondrial motility in the axon of a neuron transfected with SYP-YFP, RFP-mito, and Miro-Myc (A) or MiroKK (B). The first frame and kymograph of mitochondria labeled with RFP-mito are paired with hand-drawn traces that extract those mitochondria moving anterograde (red) or retrograde (blue). Kymographs were made before (upper) and after (lower) 5 minutes in the presence of calcimycin. (C) From kymographs as in A and B, the percent of time each mitochondrion was in motion was determined and averaged (n=145-156 mitochondria from 10 axons and 4 separate transfections per genotype). Calcimycin (Cal) inhibited both directions of movement in control and Miro-transfected, but not MiroKK-transfected axons. *, <0.05, **, <0.01, ***, <0.001, and error bars represent mean±S.E.M. here and for all figures. Scale bars, 10 μm.
Figure 4. All Axonal Mitochondria Have KHC
(A, B) In axons transfected with KHC-mCit (green), RFP-mito (magenta), milton, and Miro (A) or MiroKK (B), KHC colocalizes with mitochondria both before and after calcimycin addition. (C) Quantification of the fluorescent KHC present on mitochondria defined by the intensity of mCit in the region overlapping RFP-mito, averaged across 30-31 mitochondria from 3 independent transfections, before and after calcimycin (Cal) application. No significant change in intensity was detected upon calcimycin application (P>0.23 by Mann-Whitney U test). WT, Miro/milton/KHC-transfected neurons; KK, MiroKK/milton/KHC-transfected neurons. (D, E) In kymographs from axons like those in A and B, KHC-mCit and RFP-mito colocalize on stationary mitochondria and move congruently, regardless of the direction of motion, both before and after calcimycin addition. (F) Significant inhibition of movement by calcimycin (Cal) was found in control and Miro/milton/KHC-transfected but not in MiroKK/milton/KHC-transfected neurons. n=144-152 mitochondria from 10 axons and 4 separate transfections. (G) Ca++ does not cause dissociation of the milton/Miro/KHC complex. HEK cells were transfected with Miro or MiroKK together with KHC-ECFP and milton, and lysed in either 5mM EGTA or 2mM Ca++. KHC-ECFP was precipitated with anti-GFP and the precipitate (IP) was assayed. Input lanes were loaded with one fifth the amount of homogenate used for immunoprecipitations, and anti-tubulin was used as a loading control. (H) A mitochondrial enriched fraction from cultured rat hippocampal neurons was separated from other cytoplasmic components (Non-mito), and resuspended in either 0 or 2 mM Ca++ buffer. After centrifugation the pellet (Mito) and supernatant (Wash) were separated by SDS-PAGE and assayed for endogenous KHC, the mitochondrial marker VDAC, and the synaptic vesicle marker SYP. Scale bars, 10 μm.
Figure 5. Ca++, via Miro, Releases KHC from Microtubules
Lysates of HEK cells, transfected as indicated, were mixed with Taxol-stabilized microtubules in either 0 or 2 mM Ca++ buffer prior to sedimentation of the microtubules and microtubule-bound proteins by centrifugation (pellet, P), leaving unbound proteins in the supernatant fraction (S). Equivalent fractions of the supernatant and pellet were assayed for KHC-ECFP, milton, Miro-Myc, and tubulin.
Figure 6. Binding of the Kinesin Motor Domain to Microtubules is Inhibited by Ca++-dependent Interactions with Miro
(A) Immunoprecipitations in the presence or absence of Ca++ of KHC-ECFP (with anti-GFP) from lysates of HEK cells, transfected as indicated. In the presence of milton, neither Ca++ nor the EF-hands are required for Miro to precipitate with KHC, but in the absence of milton, both are needed (for Ca++, compare third and fourth lanes; for the EF-hands compare fourth and eighth lanes). Low levels of coprecipitation in their absence may be mediated by endogenous milton. (B, C) Illustration of the two modes of association of Miro and KHC, a Ca++-independent association via milton (B) and a Ca++-dependent, milton-independent association (C). Blue boxes represent EF-hands; the black region represents the milton binding site in KHC. (D) Immunoprecipitations with anti-Myc from cells expressing Myc-tagged full length (FL, left two panels) or truncated KHC 1-682 (682, right two panels), and T7-tagged DMiroΔTM, but not milton. (E, F) Illustration of the persistence of the Ca++-induced association of Miro and KHC, despite deletions of the KHC tail and TM domain of Miro. (G) Immunoprecipitations with anti-Myc from cells transfected with Miro-Myc and the kinesin motor domain (YFP-tagged KIF5C1-335). (H) Illustration of the dependence of motor domain/Miro interactions on Ca++ and the EF hands of Miro. (I) Microtubule cosedimentations were performed in either 0 or 2 mM Ca++ buffer and with Taxol-stabilized microtubules and lysates of the indicated transfected HEK cells. Equal volumes of supernatants (S) and microtubule pellets (P) were loaded and probed separately for KIF5C(1-335)-YFP, Miro-Myc or tubulin. (J) Illustration of the Ca++-dependent, EF-hand-dependent shift of the motor domain from microtubules to Miro, as in (G-I). (K) Ca++ dependence of the cosedimentation of the motor domain KIF5C(1-335)-YFP with microtubules, as in (I), in the presence of Miro-Myc, and the indicated free Ca++ concentrations. The level of KIF5C(1-335)-YFP in each fraction was determined with anti-GFP. (L) Ca++ dependence of the binding of Miro-Myc to KIF5C(1-335)-YFP, by precipitation from lysates of transfected HEK cells with anti-Myc and probing with anti-GFP (KIF5C(1-335)-YFP). (M) Quantification of Ca++ dependence of motor-domain cosedimentation with microtubules (as in K) and coprecipitation with Miro (as in L). The percent bound to microtubules was calculated as the intensity of the band in the pellet divided by the summed intensity of bands in both pellet and supernatant, and was defined as 100% in 0 Ca++. n=3 transfections. The percent of maximal binding to Miro was defined as the intensity of the precipitated band normalized to its input and set at 100% in 2 mM Ca++. n=3 transfections. For each section, input lanes contained one fifth of the amounts used for immunoprecipitation, and anti-tubulin was used as a loading control.
Figure 7. The EF-hands of Miro Mediate Responses of Dendritic Mitochondria to Glutamate and Protect against Excitotoxicity
(A) Mitochondrial motility in dendrites of neurons transfected with Miro-Myc (left panels), or MiroKK-Myc (right panels), together with PSD95-YFP (to mark dendrites), and RFP-mito. Kymographs of RFP-mito were made before (upper) and after (lower) a 1 min exposure to glutamate. (B) From kymographs as in A, the percent of time mitochondria were in motion was determined and averaged (n=53-61 mitochondria from 6 axons and 3 separate transfections). Glutamate (Glu) arrested bidirectional mitochondrial movement in Miro-transfected, but not MiroKK-transfected dendrites. (C) Representative 25× magnification views of neurons transfected with GFP and Miro (left two panels), or GFP and MiroKK (right two panels). After incubation with 10μM NMDA for 5 min, the neurons were either imaged directly (acute) or cultured for 24 h and then imaged. Scale bars, 100 μm. (D) Quantification of surviving neurons. NMDA (0 to 30 μM) was incubated with the transfected neurons for 5 min, and GFP positive neurons were counted 24 h later under 25× along one diameter of the coverslip. Each data point was from 10 coverslips from 3 separate transfections. Significantly different pairs are marked. (E) Schematic model of the means by which Ca++ interacts with Miro to regulate the anterograde motility of mitochondria.
Similar articles
- Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase.
Saotome M, Safiulina D, Szabadkai G, Das S, Fransson A, Aspenstrom P, Rizzuto R, Hajnóczky G. Saotome M, et al. Proc Natl Acad Sci U S A. 2008 Dec 30;105(52):20728-33. doi: 10.1073/pnas.0808953105. Epub 2008 Dec 19. Proc Natl Acad Sci U S A. 2008. PMID: 19098100 Free PMC article. - Miro proteins coordinate microtubule- and actin-dependent mitochondrial transport and distribution.
López-Doménech G, Covill-Cooke C, Ivankovic D, Halff EF, Sheehan DF, Norkett R, Birsa N, Kittler JT. López-Doménech G, et al. EMBO J. 2018 Feb 1;37(3):321-336. doi: 10.15252/embj.201696380. Epub 2018 Jan 8. EMBO J. 2018. PMID: 29311115 Free PMC article. - Ca2+-dependent regulation of mitochondrial dynamics by the Miro-Milton complex.
Liu X, Hajnóczky G. Liu X, et al. Int J Biochem Cell Biol. 2009 Oct;41(10):1972-6. doi: 10.1016/j.biocel.2009.05.013. Epub 2009 May 27. Int J Biochem Cell Biol. 2009. PMID: 19481172 Free PMC article. Review. - Interaction between the mitochondrial adaptor MIRO and the motor adaptor TRAK.
Baltrusaitis EE, Ravitch EE, Fenton AR, Perez TA, Holzbaur ELF, Dominguez R. Baltrusaitis EE, et al. J Biol Chem. 2023 Dec;299(12):105441. doi: 10.1016/j.jbc.2023.105441. Epub 2023 Nov 8. J Biol Chem. 2023. PMID: 37949220 Free PMC article. - Regulation of long-distance transport of mitochondria along microtubules.
Melkov A, Abdu U. Melkov A, et al. Cell Mol Life Sci. 2018 Jan;75(2):163-176. doi: 10.1007/s00018-017-2590-1. Epub 2017 Jul 12. Cell Mol Life Sci. 2018. PMID: 28702760 Free PMC article. Review.
Cited by
- Progressive Decrease of Mitochondrial Motility during Maturation of Cortical Axons In Vitro and In Vivo.
Lewis TL Jr, Turi GF, Kwon SK, Losonczy A, Polleux F. Lewis TL Jr, et al. Curr Biol. 2016 Oct 10;26(19):2602-2608. doi: 10.1016/j.cub.2016.07.064. Epub 2016 Sep 15. Curr Biol. 2016. PMID: 27641765 Free PMC article. - Impaired Mitochondrial Dynamics and Mitophagy in Neuronal Models of Tuberous Sclerosis Complex.
Ebrahimi-Fakhari D, Saffari A, Wahlster L, Di Nardo A, Turner D, Lewis TL Jr, Conrad C, Rothberg JM, Lipton JO, Kölker S, Hoffmann GF, Han MJ, Polleux F, Sahin M. Ebrahimi-Fakhari D, et al. Cell Rep. 2016 Oct 18;17(4):1053-1070. doi: 10.1016/j.celrep.2016.09.054. Cell Rep. 2016. PMID: 27760312 Free PMC article. - Axon diameter and axonal transport: In vivo and in vitro effects of androgens.
Pesaresi M, Soon-Shiong R, French L, Kaplan DR, Miller FD, Paus T. Pesaresi M, et al. Neuroimage. 2015 Jul 15;115:191-201. doi: 10.1016/j.neuroimage.2015.04.048. Epub 2015 May 6. Neuroimage. 2015. PMID: 25956809 Free PMC article. - Mitophagy and Oxidative Stress: The Role of Aging.
De Gaetano A, Gibellini L, Zanini G, Nasi M, Cossarizza A, Pinti M. De Gaetano A, et al. Antioxidants (Basel). 2021 May 17;10(5):794. doi: 10.3390/antiox10050794. Antioxidants (Basel). 2021. PMID: 34067882 Free PMC article. Review. - DISC1 is a coordinator of intracellular trafficking to shape neuronal development and connectivity.
Devine MJ, Norkett R, Kittler JT. Devine MJ, et al. J Physiol. 2016 Oct 1;594(19):5459-69. doi: 10.1113/JP272187. Epub 2016 Jun 12. J Physiol. 2016. PMID: 27121900 Free PMC article. Review.
References
- Adio S, Reth J, Bathe F, Woehlke G. Regulation mechanisms of Kinesin-1. J Muscle Res Cell Motil. 2006;27:153–160. - PubMed
- Bader MF, Doussau F, Chasserot-Golaz S, Vitale N, Gasman S. Coupling actin and membrane dynamics during calcium-regulated exocytosis: a role for Rho and ARF GTPases. Biochim Biophys Acta. 2004;1742:37–49. - PubMed
- Beck M, Brickley K, Wilkinson HL, Sharma S, Smith M, Chazot PL, Pollard S, Stephenson FA. Identification, molecular cloning, and characterization of a novel GABAA receptor-associated protein, GRIF-1. J Biol Chem. 2002;277:30079–30090. - PubMed
- Boldogh IR, Pon LA. Mitochondria on the move. Trends Cell Biol. 2007;17:502–510. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous