ClpX inhibits FtsZ assembly in a manner that does not require its ATP hydrolysis-dependent chaperone activity - PubMed (original) (raw)
ClpX inhibits FtsZ assembly in a manner that does not require its ATP hydrolysis-dependent chaperone activity
Daniel P Haeusser et al. J Bacteriol. 2009 Mar.
Abstract
ClpX is a well-characterized bacterial chaperone that plays a role in many processes, including protein turnover and the remodeling of macromolecular complexes. All of these activities require ATP hydrolysis-dependent, ClpX-mediated protein unfolding. Here we used site-directed mutagenesis in combination with genetics and biochemistry to establish that ClpX inhibits assembly of the conserved division protein FtsZ through a noncanonical mechanism independent of its role as an ATP-dependent chaperone.
Figures
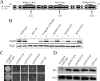
FIG. 1.
Mutations in B. subtilis ClpX residues predicted to be required for interaction with canonical substrates disrupt ClpXP degradation of Spx. (A) Alignment of B. subtilis and E. coli ClpX polypeptides. Amino acid identity is indicated by black boxes and amino acid similarity by gray boxes. Bars indicate Walker A, pore loop, and Walker B boxes, respectively. Numbers refer to the B. subtilis ClpX sequence. Arrows indicate residues that were changed to alanine for this study. (B) Quantitative immunoblot of lysates from mid-exponential-phase cultures grown in the absence (−) or presence (+) of xylose. In strains encoding clpX under the control of both its native promoter and the exogenous Pxyl promoter, ClpX levels were ∼2-fold lower than those in the parent strain in both the mutant and congenic wild-type backgrounds in the absence of xylose. Xylose-induced expression from the Pxyl promoter raised the intracellular concentration of ClpX to wild-type levels in these strains. Lysates were blotted with anti-ClpX sera (top) or anti-FtsZ sera (bottom). ClpX appears as a doublet on immunoblots. Mutations in clpX do not affect FtsZ levels. (C) Colonies stabbed onto tryptone broth swarm plates in the presence and absence of xylose. Note that only the congenic wild-type strain is motile, indicating appropriate turnover of Spx. Minor differences in swarm size in the congenic wild-type strain are most likely the result of experimental variation and are not a reflection of changes in ClpX levels. (D) Immunoblot of lysates from mid-exponential cultures probed with antisera against the ClpX target Spx or with anti-FtsZ sera. Note the increased levels of Spx in the four clpX mutant strains, confirming a defect in the ClpX-Spx interaction. FtsZ serves as a loading control.
FIG. 2.
Mutations in residues required for ClpXP-mediated proteolysis have a negligible effect on the ability of ClpX to inhibit FtsZ assembly. (A) Suppression of the lethality associated with >12-fold overexpression of the MinCD division inhibitor. Both the Walker B (E182A) and pore loop (Y150A) mutants are sensitive to MinCD overexpression in the presence or absence of xylose, indicating that they are wild type with regard to inhibiting FtsZ assembly. In contrast, the predicted ClpX Walker A mutant (K122A) is sensitive to high levels of MinCD only when expressed at wild-type levels in the presence of xylose. A twofold reduction in the ClpX(K122A) concentration by growth in the absence of xylose results in an increase in resistance to MinCD overexpression. Error bars indicate standard deviations from at least three separate experiments. (B) Representative traces of 90°-angle light-scattering reactions containing 3 μM FtsZ assembled alone or in the presence of wild-type ClpX (top) or a mutant that is defective in ATP hydrolysis, ClpX(E182A). (C) Histogram of FtsZ assembly reactions conducted in the presence of the wild-type or mutant ClpX protein. Each bar represents at least three replicate experiments. Error bars indicate standard deviations from three separate experiments.
Similar articles
- The ClpX chaperone modulates assembly of the tubulin-like protein FtsZ.
Weart RB, Nakano S, Lane BE, Zuber P, Levin PA. Weart RB, et al. Mol Microbiol. 2005 Jul;57(1):238-49. doi: 10.1111/j.1365-2958.2005.04673.x. Mol Microbiol. 2005. PMID: 15948963 Free PMC article. - AAA+ chaperone ClpX regulates dynamics of prokaryotic cytoskeletal protein FtsZ.
Sugimoto S, Yamanaka K, Nishikori S, Miyagi A, Ando T, Ogura T. Sugimoto S, et al. J Biol Chem. 2010 Feb 26;285(9):6648-57. doi: 10.1074/jbc.M109.080739. Epub 2009 Dec 17. J Biol Chem. 2010. PMID: 20022957 Free PMC article. - The interplay of ClpXP with the cell division machinery in Escherichia coli.
Camberg JL, Hoskins JR, Wickner S. Camberg JL, et al. J Bacteriol. 2011 Apr;193(8):1911-8. doi: 10.1128/JB.01317-10. Epub 2011 Feb 11. J Bacteriol. 2011. PMID: 21317324 Free PMC article. - Chaperone-protease systems in regulation and protein quality control in Bacillus subtilis.
Molière N, Turgay K. Molière N, et al. Res Microbiol. 2009 Nov;160(9):637-44. doi: 10.1016/j.resmic.2009.08.020. Epub 2009 Sep 23. Res Microbiol. 2009. PMID: 19781636 Review. - A camel passes through the eye of a needle: protein unfolding activity of Clp ATPases.
Zolkiewski M. Zolkiewski M. Mol Microbiol. 2006 Sep;61(5):1094-100. doi: 10.1111/j.1365-2958.2006.05309.x. Mol Microbiol. 2006. PMID: 16879409 Free PMC article. Review.
Cited by
- Connecting sequence features within the disordered C-terminal linker of Bacillus subtilis FtsZ to functions and bacterial cell division.
Shinn MK, Cohan MC, Bullock JL, Ruff KM, Levin PA, Pappu RV. Shinn MK, et al. Proc Natl Acad Sci U S A. 2022 Oct 18;119(42):e2211178119. doi: 10.1073/pnas.2211178119. Epub 2022 Oct 10. Proc Natl Acad Sci U S A. 2022. PMID: 36215496 Free PMC article. - The Division Defect of a Bacillus subtilis minD noc Double Mutant Can Be Suppressed by Spx-Dependent and Spx-Independent Mechanisms.
Yu Y, Dempwolff F, Oshiro RT, Gueiros-Filho FJ, Jacobson SC, Kearns DB. Yu Y, et al. J Bacteriol. 2021 Aug 20;203(18):e0024921. doi: 10.1128/JB.00249-21. Epub 2021 Aug 20. J Bacteriol. 2021. PMID: 34181483 Free PMC article. - β-Lactams against the Fortress of the Gram-Positive Staphylococcus aureus Bacterium.
Fisher JF, Mobashery S. Fisher JF, et al. Chem Rev. 2021 Mar 24;121(6):3412-3463. doi: 10.1021/acs.chemrev.0c01010. Epub 2020 Dec 29. Chem Rev. 2021. PMID: 33373523 Free PMC article. Review. - Degradation of MinD oscillator complexes by Escherichia coli ClpXP.
LaBreck CJ, Trebino CE, Ferreira CN, Morrison JJ, DiBiasio EC, Conti J, Camberg JL. LaBreck CJ, et al. J Biol Chem. 2021 Jan-Jun;296:100162. doi: 10.1074/jbc.RA120.013866. Epub 2020 Dec 10. J Biol Chem. 2021. PMID: 33288679 Free PMC article. - ClpX Is Essential and Activated by Single-Strand DNA Binding Protein in Mycobacteria.
Kester JC, Kandror O, Akopian T, Chase MR, Zhu J, Rubin EJ, Goldberg AL, Fortune SM. Kester JC, et al. J Bacteriol. 2021 Jan 25;203(4):e00608-20. doi: 10.1128/JB.00608-20. Print 2021 Jan 25. J Bacteriol. 2021. PMID: 33229461 Free PMC article.
References
- Banecki, B., A. Wawrzynow, J. Puzewicz, C. Georgopoulos, and M. Zylicz. 2001. Structure-function analysis of the zinc-binding region of the ClpX molecular chaperone. J. Biol. Chem. 27618843-18848. - PubMed
- Burton, B. M., and T. A. Baker. 2003. Mu transpososome architecture ensures that unfolding by ClpX or proteolysis by ClpXP remodels but does not destroy the complex. Chem. Biol. 10463-472. - PubMed
- Burton, B. M., T. L. Williams, and T. A. Baker. 2001. ClpX-mediated remodeling of mu transpososomes: selective unfolding of subunits destabilizes the entire complex. Mol. Cell 8449-454. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources