Yeast Mph1 helicase dissociates Rad51-made D-loops: implications for crossover control in mitotic recombination - PubMed (original) (raw)
Yeast Mph1 helicase dissociates Rad51-made D-loops: implications for crossover control in mitotic recombination
Rohit Prakash et al. Genes Dev. 2009.
Abstract
Eukaryotes possess mechanisms to limit crossing over during homologous recombination, thus avoiding possible chromosomal rearrangements. We show here that budding yeast Mph1, an ortholog of human FancM helicase, utilizes its helicase activity to suppress spontaneous unequal sister chromatid exchanges and DNA double-strand break-induced chromosome crossovers. Since the efficiency and kinetics of break repair are unaffected, Mph1 appears to channel repair intermediates into a noncrossover pathway. Importantly, Mph1 works independently of two other helicases-Srs2 and Sgs1-that also attenuate crossing over. By chromatin immunoprecipitation, we find targeting of Mph1 to double-strand breaks in cells. Purified Mph1 binds D-loop structures and is particularly adept at unwinding these structures. Importantly, Mph1, but not a helicase-defective variant, dissociates Rad51-made D-loops. Overall, the results from our analyses suggest a new role of Mph1 in promoting the noncrossover repair of DNA double-strand breaks.
Figures
Figure 1.
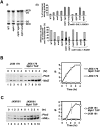
Screen for genes regulating the crossover frequency identifies Mph1. (A) Ectopic gene conversion is induced by DSBs generated by the HO endonuclease within a 1.9-kb MAT a sequence (gray rectangle) that replaced the ARG5,6 gene on chromosome V. The MAT a-inc sequence on chromosome III is a donor for recombination, and shares 1403-bp and 530-bp homology on opposite sides of the DSB. EcoRI noncrossover fragments (NCOs) of 6.4 kb and 3 kb can be distinguished from crossover fragments (COs) of 6 kb and 3.4 kb, and quantified on Southern blots. The probe used to detect crossovers and noncrossovers was a MAT a fragment overlapping the first 200 bp on each side of the HO break. (B) Viability resulting from DSB repaired by ectopic recombination was measured by dividing colony-forming units on YEP-galactose over those on YEP-dextrose. Crossover frequency among the product in SRS2 synthetic interactors was determined 8 h after break induction as the intensity ratio of the Southern blot signal corresponding to gene conversion with crossover and that corresponding to gene conversion both with and without crossover.
Figure 2.
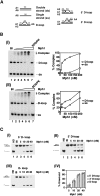
MPH1 helicase channels DSB repair to noncrossovers. (A) Kinetics of DSB repair in wild type and _mph1_Δ mutant, determined by dividing the normalized Southern blot signals corresponding to product at different times by the signal corresponding to the maximal product at 8 h after break induction in wild-type cells. (B) Comparison of growth rate between _srs2_Δ and _mph1_Δ. Slow growth rate of double mutant _srs2_Δ _mph1_Δ is suppressed by elimination of RAD51. (C) Crossover level among products in _mph1_Δ and helicase point mutants is shown. The _mph1_Δ strain was complemented with plasmids carrying either wild-type or mutant MPH1 sequences.
Figure 3.
Mph1 is recruited to DSBs, and works independently of Sgs1 and Srs2. (A, panel I) Southern blot analysis of gene conversion with and without crossovers in strains lacking the indicated helicases. (Panel II, top) Percentage of crossovers in cells that repaired the DSB. (Bottom) Percentage of crossovers (black) and noncrossovers (gray) among all cells with the HO cut. These percentages were determined by dividing the normalized intensities of the crossover or noncrossover band on Southern blot by the intensity of parental uncut MATa band before break induction (time, 0 h) (B) Time-course ChIP experiments showing recruitment of TAP-tagged Mph1 to nonrepairable HO break (strain JKM179) or to the HMLα donor sequence in strain (JKM 161) that can repair the break by gene conversion.
Figure 4.
DNA binding and D-loop unwinding by Mph1. (A) DNA substrates used for DNA-binding and DNA-unwinding assays. The oligonucleotides used for constructing the substrates and the sizes of the DNA regions in the substrates are indicated and their sequences are given in Supplemental Table 2. In the central portion of the D-loop substrates, the unpaired strand bears no homology with the paired duplex region. (B) Mph1 (10–200 nM) was incubated with the 5′ D-loop substrate (50 nM) and dsDNA (50 nM) (panel I) or ssDNA (50 nM) (panel II). The results from these DNA mobility shift experiments were plotted. (C) Mph1 (5 to 40 nM) was incubated with D-loop substrates (50 nM each) that harbored a 5′ tail (panel I), a 3′ tail (panel II), or no tail (panel III). The heat-denatured substrate (HD) was run in lane 1 and the reaction blank was run in lane 2. The results from these DNA unwinding experiments were plotted in panel IV.
Figure 5.
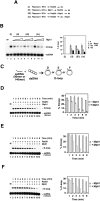
Effect of Mph1 on the Rad51-mediated D-loop reaction. (A) Schematic of the D-loop reaction. The ssDNA substrate was the 90-mer Oligonucleotide D1, homologous to positions 1932–2022 of pBluescript SK DNA (see Supplemental Table 2 for detailed sequence). (B) D-loop reactions without (lane 2) or with (lanes 3_–_6) Mph1 (50, 100, 150, and 200 nM) were analyzed after 4 or 8 min of incubation. The reaction blank (Bl) was run in lane 1. The results were plotted. (C) D-loop reactions without (lane 2) or with (lanes 3_–_6) mph1 D209N (50, 100, 150, and 200 nM) were analyzed after 8 min of incubation. The reaction blank (Bl) was run in lane 1. The results were plotted. The concentration of Rad51 was 0.8 μM and of Oligonucleotide D1 was 2.4 μM (nucleotides).
Figure 6.
Mph1 acts by dissociating preformed D-loops. (A) Summary of D-loop reactions that did not contain Mph1 (i) or with Mph1 (50, 100, 200 nM) added at different stages (ii–iv). The reactions used Oligonucleotide D1 as the ssDNA substrate. (B) Results from the reactions summarized in A. The reaction blank was run in lane 1. The results were plotted. (C) Schematic of D-loop reactions utilizing ssDNA substrates that gave no tail (90-mer Oligonucleotide D1), a 50-nucleotide 3′ tail (the 140-mer Oligonucleotide D2), or a 50-nucleotide 5′ tail (the 140-mer Oligonucleotide D3). All three oligonucleotides bear homology with positions 1932 to 2022 of pBluescript SK DNA and their sequence is given in Supplemental Table 2. (D–F) Time course experiments that examined formation the D-loops with no tail (D), a 3′ 50-nucleotide tail (E), or a 5′ 50-nucleotide tail (F). These reactions either contained Mph1 (150 nM; lanes 8–13) or not (lanes 2_–_7). In D, 2.4 μM (nucleotides) of Oligonucleotide D1 and 0.8 μM of Rad51 were used; in E and F, 3.72 μM of Oligonucleotide D2 or D3 and 1.24 μM of Rad51 were used. The reaction blank (Bl) was run in lane 1. The results were plotted.
Figure 7.
Mechanism and specificity of Mph1 action. (A) In I, Mph1 or Srs2 (50 or 100 nM) was added with Rad51 at the beginning of the D-loop reaction, which, following the incorporation of pBluescript SK DNA, was incubated for 8 min. The reaction blank and the reaction without any helicase were run in lanes 1 and 2, respectively. The results were plotted. In II, Mph1 or Srs2 (50 or 100 nM) was added to the D-loop reaction 1 min after product synthesis had commenced, followed by an additional 8-min incubation. The reaction blank and the reaction without any helicase were run in lanes 1 and 2, respectively. The results were plotted. Oligonucleotide D1 was used at 2.4 μM (nucleotides) and Rad51 was at 0.8 μM, as in Figure 6. (B) Mph1, BLM, RecQ1, and WRN (50 or 100 nM) were tested for their ability to dissociate human Rad51-made D-loops that harbored no tail (I), a 3′ tail (II), or a 5′ tail (III). The D-loops were generated with Oligonucleotide D1 (2.4 μM nucleotides with 0.8 μM Rad51), D2 (3.72 μM nucleotides with 1.24 μM Rad51), or D3 (3.73 μM nucleotides with 1.24 μM Rad51) and pBluescript SK duplex DNA, as in Figure 6. The helicases were added with Rad51 at the beginning of the reaction and the incubation time was 8 min. The reaction blank and the reaction without any helicase were run in lanes 1 and 2, respectively. The results were plotted.
Figure 8.
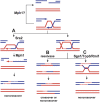
Model depicting regulation of exchange frequency by DNA helicases during DSB-induced recombination. (A) Both Mph1 and Srs2 promote the noncrossover SDSA pathway by minimizing the possibility of creating a dHJ. Mph1 displaces the invading strand after DNA synthesis has commenced, thus preventing dHJ formation and promoting SDSA. Mph1 may also inhibit recombination if it dissociates D-loop before invading strand-initiated DNA synthesis. Srs2 possibly removes Rad51 from the unpaired 3′ DNA tail to prevent second end capture and dHJ formation. (B) When the D-loop becomes extended and more stable, dHJs are formed and can be resolved into crossovers and noncrossovers by a yet-unknown resolvase. (C) Sgs1 helicase in complex with Rmi1 and Top3 resolves these mitotic dHJs into noncrossovers.
Similar articles
- DNA Helicase Mph1FANCM Ensures Meiotic Recombination between Parental Chromosomes by Dissociating Precocious Displacement Loops.
Sandhu R, Monge Neria F, Monge Neria J, Chen X, Hollingsworth NM, Börner GV. Sandhu R, et al. Dev Cell. 2020 May 18;53(4):458-472.e5. doi: 10.1016/j.devcel.2020.04.010. Epub 2020 May 7. Dev Cell. 2020. PMID: 32386601 Free PMC article. - Heteroduplex DNA position defines the roles of the Sgs1, Srs2, and Mph1 helicases in promoting distinct recombination outcomes.
Mitchel K, Lehner K, Jinks-Robertson S. Mitchel K, et al. PLoS Genet. 2013;9(3):e1003340. doi: 10.1371/journal.pgen.1003340. Epub 2013 Mar 14. PLoS Genet. 2013. PMID: 23516370 Free PMC article. - The Srs2 helicase activity is stimulated by Rad51 filaments on dsDNA: implications for crossover incidence during mitotic recombination.
Dupaigne P, Le Breton C, Fabre F, Gangloff S, Le Cam E, Veaute X. Dupaigne P, et al. Mol Cell. 2008 Feb 1;29(2):243-54. doi: 10.1016/j.molcel.2007.11.033. Mol Cell. 2008. PMID: 18243118 - The role of homologous recombination repair in the formation of chromosome aberrations.
Griffin CS, Thacker J. Griffin CS, et al. Cytogenet Genome Res. 2004;104(1-4):21-7. doi: 10.1159/000077462. Cytogenet Genome Res. 2004. PMID: 15162011 Review. - Emerging non-canonical roles for the Rad51-Rad52 interaction in response to double-strand breaks in yeast.
Ngo K, Epum EA, Friedman KL. Ngo K, et al. Curr Genet. 2020 Oct;66(5):917-926. doi: 10.1007/s00294-020-01081-z. Epub 2020 May 12. Curr Genet. 2020. PMID: 32399607 Free PMC article. Review.
Cited by
- Detection of Homologous Recombination Intermediates via Proximity Ligation and Quantitative PCR in Saccharomyces cerevisiae.
Reitz D, Savocco J, Piazza A, Heyer WD. Reitz D, et al. J Vis Exp. 2022 Sep 11;(187):10.3791/64240. doi: 10.3791/64240. J Vis Exp. 2022. PMID: 36155960 Free PMC article. - Investigations of homologous recombination pathways and their regulation.
Daley JM, Kwon Y, Niu H, Sung P. Daley JM, et al. Yale J Biol Med. 2013 Dec 13;86(4):453-61. Yale J Biol Med. 2013. PMID: 24348209 Free PMC article. Review. - DNA Helicase Mph1FANCM Ensures Meiotic Recombination between Parental Chromosomes by Dissociating Precocious Displacement Loops.
Sandhu R, Monge Neria F, Monge Neria J, Chen X, Hollingsworth NM, Börner GV. Sandhu R, et al. Dev Cell. 2020 May 18;53(4):458-472.e5. doi: 10.1016/j.devcel.2020.04.010. Epub 2020 May 7. Dev Cell. 2020. PMID: 32386601 Free PMC article. - Main steps in DNA double-strand break repair: an introduction to homologous recombination and related processes.
Ranjha L, Howard SM, Cejka P. Ranjha L, et al. Chromosoma. 2018 Jun;127(2):187-214. doi: 10.1007/s00412-017-0658-1. Epub 2018 Jan 11. Chromosoma. 2018. PMID: 29327130 Review. - Restriction of replication fork regression activities by a conserved SMC complex.
Xue X, Choi K, Bonner JN, Chiba T, Kwon Y, Xu Y, Sanchez H, Wyman C, Niu H, Zhao X, Sung P. Xue X, et al. Mol Cell. 2014 Nov 6;56(3):436-445. doi: 10.1016/j.molcel.2014.09.013. Epub 2014 Oct 16. Mol Cell. 2014. PMID: 25439736 Free PMC article.
References
- Allers T., Lichten M. Differential timing and control of noncrossover and crossover recombination during meiosis. Cell. 2001;106:47–57. - PubMed
- Bridge W.L., Vandenberg C.J., Franklin R.J., Hiom K. The BRIP1 helicase functions independently of BRCA1 in the Fanconi anemia pathway for DNA crosslink repair. Nat. Genet. 2005;37:953–957. - PubMed
- Brosh R.M., Jr, Orren D.K., Nehlin J.O., Ravn P.H., Kenny M.K., Machwe A., Bohr V.A. Functional and physical interaction between WRN helicase and human replication protein A. J. Biol. Chem. 1999;274:18341–18350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM020056-32/GM/NIGMS NIH HHS/United States
- R01 GM053738/GM/NIGMS NIH HHS/United States
- R01 GM076020/GM/NIGMS NIH HHS/United States
- GM53738/GM/NIGMS NIH HHS/United States
- GM20056/GM/NIGMS NIH HHS/United States
- GM57814/GM/NIGMS NIH HHS/United States
- R01 GM076020-04/GM/NIGMS NIH HHS/United States
- GR076476/PHS HHS/United States
- R01 GM080600-03/GM/NIGMS NIH HHS/United States
- R01 GM057814/GM/NIGMS NIH HHS/United States
- R01 GM080600/GM/NIGMS NIH HHS/United States
- R37 GM020056/GM/NIGMS NIH HHS/United States
- GM80600/GM/NIGMS NIH HHS/United States
- ES07061/ES/NIEHS NIH HHS/United States
- R01 GM020056-34/GM/NIGMS NIH HHS/United States
- R01 GM020056-33/GM/NIGMS NIH HHS/United States
- R01 ES007061/ES/NIEHS NIH HHS/United States
- R01 GM020056/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials