Sex and depot differences in adipocyte insulin sensitivity and glucose metabolism - PubMed (original) (raw)
Sex and depot differences in adipocyte insulin sensitivity and glucose metabolism
Yazmin Macotela et al. Diabetes. 2009 Apr.
Abstract
Objective: To investigate how insulin sensitivity and glucose metabolism differ in adipocytes between different fat depots of male and female mice and how sex steroids contribute to these differences.
Research design and methods: Adipocytes from intra-abdominal/perigonadal (PG) and subcutaneous (SC) adipose tissue from normal, castrated, or steroid-implanted animals were isolated and analyzed for differences in insulin sensitivity and glucose metabolism.
Results: Adipocytes from both PG and SC depots of females have increased lipogenic rates compared with those from males. In females, intra-abdominal PG adipocytes are more insulin-sensitive than SC adipocytes and more insulin-sensitive than male adipocytes from either depot. When stimulated by low physiological concentrations of insulin, female PG adipocytes show a robust increase in Akt and extracellular signal-related kinase (ERK) phosphorylation and lipogenesis, whereas male adipocytes show activation only at higher insulin concentrations. Adipocytes from females have higher mRNA/protein levels of several genes involved in glucose and lipid metabolism. After castration, adipocytes of male mice showed increased insulin sensitivity and increased lipogenic rates, whereas adipocytes of females demonstrate decreased lipid production. Increasing estrogen above physiological levels, however, also reduced lipid synthesis in females, whereas increasing dihydrotestosterone in males had no effect.
Conclusions: There are major sex differences in insulin sensitivity in adipose tissue, particularly in the intra-abdominal depot, that are regulated by physiological levels of sex steroids. The increased sensitivity to insulin and lipogenesis observed in adipocytes from females may account for their lower level of insulin resistance and diabetes risk despite similar or higher fat content than in males.
Figures
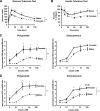
FIG. 1.
Female mice and female adipocytes have increased insulin sensitivity. Glucose tolerance test was performed after an overnight fast. Mice received an intraperitoneal injection of 2 g/kg body wt glucose. Blood glucose was measured from tail vein samples at the indicated times (A). Insulin tolerance test was performed on random-fed mice. Animals received injections of 1 unit/kg body wt insulin. Blood glucose was measured at the indicated time points (B). Data points are means ± SE with eight mice in each group. Adipocytes from PG and SC fat were isolated from male and female mice and stimulated with or without insulin in the presence of 14C-glucose for 90 min as described in
research design and methods
. Radioactive glucose incorporated into lipids was measured for each condition in the extracted lipids and normalized to total lipid content (C) or to cell number (D) as determined by counting of osmic acid fixed cells, n = 3. **P < 0.01 by repeated-measures ANOVA. *P < 0.05 by Student's t test for point-to-point comparisons.
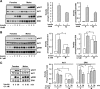
FIG. 2.
Increased insulin-stimulated Akt and ERK activation in female adipocytes. Adipocytes from PG fat isolated from male and female mice were treated with or without 10 nmol/l (A) or 0.1 nmol/l (B and C) insulin for 5 (A–C) or 30 min (C). Cell lysates were run on SDS-PAGE and subjected to Western blot analysis using antibodies directed against phosphorylated (pAkt Ser473, pERK Thr202/Tyr204) or total Akt and ERK. Each lane represents a pool of adipocytes from three to four mice. The data were also quantified by densitometric scanning of the autoradiographs (n = 2). *P < 0.05-fold stimulation females versus males.
FIG. 3.
Female adipocytes have increased mRNA protein expression of lipid and glucose metabolism genes. Adipocytes from PG and SC fat isolated from male and female mice were lysed by homogenization using a denaturing guanidine-thiocyanate–containing buffer, mRNA was extracted, and quantitative real-time PCR was performed (A). Gene expression for GLUT1, GLUT4, FAS, and ACC mRNAs were normalized against the expression of TATA-binding protein. B: Proteins from similar adipocyte isolates were extracted and subjected to SDS-PAGE and Western blotted with antibodies to each of these proteins. Actin was used as a control for protein loading. Each lane represents a pool of adipocytes from three to four mice. C: The data were quantified by densitometry of immunoblots (n = 3) and normalized against actin. *P < 0.05.
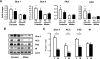
FIG. 4.
Castration reverses lipogenic rates in male and female adipocytes. Male and female mice were castrated or sham operated. Six weeks after surgery, adipocytes from PG (A and B) and SC (C and D) fat were isolated and stimulated with or without insulin in the presence of 14C-glucose for 90 min. Radioactivity was measured in the extracted lipids and normalized by total lipid content. n = 6 animals per group. **P < 0.05 by repeated-measures ANOVA. *P < 0.05 by Student's t test for point-to-point comparisons sham versus castrated.
FIG. 5.
Castration reverses gene expression patterns in male and female adipocytes. Male and female mice were castrated (C) or sham operated (S). Six weeks after surgery, adipocytes from PG and SC fat were isolated, mRNA was extracted, and quantitative real-time PCR was performed (A). Gene expression for metabolic genes was assessed and normalized against the expression of TATA-binding protein. B: Proteins from similar cells were subjected to SDS-PAGE and Western blot with the respective antibodies. Actin was used as a control for protein loading. C: Quantitation of the proteins was achieved by densitometry normalized by the values for actin. *P < 0.05. IR, insulin receptor.
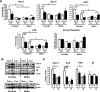
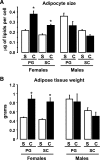
FIG. 6.
Effect of castration on adipocyte size and adipose tissue weight. Male and female mice were castrated (C) or sham operated (S). Six weeks after surgery, total body weights and adipose tissue weights from PG and SC fat were assessed, and adipocytes were isolated as described in
research design and methods.
Mean cell size was calculated by dividing the total lipid content by the total number of cells on a sample. Graphs show the mean adipocyte size (A) or adipose tissue weight (B) from six animals per group. *P < 0.05.
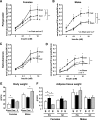
FIG. 7.
Effect of short-term castration and steroid treatment on lipogenesis, body weight, and adipose tissue weight in males versus females. Male and female mice were castrated, implanted with 17β-estradiol or 5α-DHT pellets, or sham operated. Three weeks after surgery, adipocytes from PG (A and B) and SC (C and D) fat were isolated and stimulated with or without insulin in the presence of 14C-glucose for 90 min. Radioactivity was measured in the extracted lipids and normalized by total lipid content. E and F: Total body weight (E) and adipose tissue weights (F) from PG and SC fat from sham-operated (sham or S) castrated (cast or C) implanted with estrogen-containing pellets (E) or DHT-containing pellets (T) were assessed. The graphs show the mean from eight animals per group. **P < 0.05 by repeated-measures ANOVA. *P < 0.05 by Student's t test for point-to-point comparisons.
Similar articles
- Incorporation of glucose into lipid of perirenal and subcutaneous adipocytes of rats and sheep: influence of insulin.
Broad TE, Sedcole JR, Ngan AS. Broad TE, et al. Aust J Biol Sci. 1983;36(2):147-56. doi: 10.1071/bi9830147. Aust J Biol Sci. 1983. PMID: 6354162 - Sex difference in insulin-stimulated glucose transport in rat and human adipocytes.
Foley JE, Kashiwagi A, Chang H, Huecksteadt TP, Lillioja S, Verso MA, Reaven G. Foley JE, et al. Am J Physiol. 1984 Mar;246(3 Pt 1):E211-5. doi: 10.1152/ajpendo.1984.246.3.E211. Am J Physiol. 1984. PMID: 6367483 - Increased insulin sensitivity and responsiveness of glucose metabolism in adipocytes from female versus male rats.
Guerre-Millo M, Leturque A, Girard J, Lavau M. Guerre-Millo M, et al. J Clin Invest. 1985 Jul;76(1):109-16. doi: 10.1172/JCI111932. J Clin Invest. 1985. PMID: 3894416 Free PMC article. - Lipid metabolism in women.
Williams CM. Williams CM. Proc Nutr Soc. 2004 Feb;63(1):153-60. doi: 10.1079/PNS2003314. Proc Nutr Soc. 2004. PMID: 15070445 Review. - Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids.
Smith U, Kahn BB. Smith U, et al. J Intern Med. 2016 Nov;280(5):465-475. doi: 10.1111/joim.12540. Epub 2016 Oct 3. J Intern Med. 2016. PMID: 27699898 Free PMC article. Review.
Cited by
- Sex-specific alterations in glucose homeostasis and metabolic parameters during ageing of caspase-2-deficient mice.
Wilson CH, Nikolic A, Kentish SJ, Shalini S, Hatzinikolas G, Page AJ, Dorstyn L, Kumar S. Wilson CH, et al. Cell Death Discov. 2016 Feb 29;2:16009. doi: 10.1038/cddiscovery.2016.9. eCollection 2016. Cell Death Discov. 2016. PMID: 27551503 Free PMC article. - Pancreas morphogenesis and homeostasis depends on tightly regulated Zeb1 levels in epithelial cells.
Lasierra Losada M, Pauler M, Vandamme N, Goossens S, Berx G, Leppkes M, Schuhwerk H, Brabletz S, Brabletz T, Stemmler MP. Lasierra Losada M, et al. Cell Death Discov. 2021 Jun 11;7(1):138. doi: 10.1038/s41420-021-00522-z. Cell Death Discov. 2021. PMID: 34112759 Free PMC article. - Behavioral tests of the insulin-cholinergic-dopamine link in nucleus accumbens and inhibition by high fat-high sugar diet in male and female rats.
Weiner SP, Carr KD. Weiner SP, et al. Physiol Behav. 2024 Oct 1;284:114647. doi: 10.1016/j.physbeh.2024.114647. Epub 2024 Jul 25. Physiol Behav. 2024. PMID: 39067780 - DNA microarray analysis reveals differential gene expression in the soleus muscle between male and female rats exposed to a high fat diet.
Oh TS, Yun JW. Oh TS, et al. Mol Biol Rep. 2012 Jun;39(6):6569-80. doi: 10.1007/s11033-012-1486-2. Epub 2012 Feb 4. Mol Biol Rep. 2012. PMID: 22307788 - Consideration of sex as a biological variable in diabetes research across twenty years.
Cherian CM, Reeves HR, De Silva D, Tsao S, Marshall KE, Rideout EJ. Cherian CM, et al. Biol Sex Differ. 2024 Feb 26;15(1):19. doi: 10.1186/s13293-024-00595-2. Biol Sex Differ. 2024. PMID: 38409052 Free PMC article.
References
- Lean ME: Pathophysiology of obesity. Proc Nutr Soc 59: 331– 336, 2000 - PubMed
- Mauriege P, Despres JP, Moorjani S, Prud'Homme D, Lamarche B, Bouchard C, Nadeau A, Tremblay A, Lupien PJ: Abdominal and femoral adipose tissue lipolysis and cardiovascular disease risk factors in men. Eur J Clin Invest 23: 729– 740, 1993 - PubMed
- Gillum RF: The association of body fat distribution with hypertension, hypertensive heart disease, coronary heart disease, diabetes and cardiovascular risk factors in men and women aged 18–79 years. J Chronic Dis 40: 421– 428, 1987 - PubMed
- Kissebah AH, Krakower GR: Regional adiposity and morbidity. PMID 74: 761– 811, 1994 - PubMed
- Nuutila P, Knuuti MJ, Maki M, Laine H, Ruotsalainen U, Teras M, Haaparanta M, Solin O, Yki-Jarvinen H: Gender and insulin sensitivity in the heart and in skeletal muscles: studies using positron emission tomography. Diabetes 44: 31– 36, 1995 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5P30 DK 36836/DK/NIDDK NIH HHS/United States
- P30 DK036836/DK/NIDDK NIH HHS/United States
- DK31036/DK/NIDDK NIH HHS/United States
- R01 DK033201/DK/NIDDK NIH HHS/United States
- R01 DK031036/DK/NIDDK NIH HHS/United States
- D55545/PHS HHS/United States
- DK33201/DK/NIDDK NIH HHS/United States
- R37 DK031036/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous