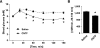Ciliary neurotrophic factor stimulates muscle glucose uptake by a PI3-kinase-dependent pathway that is impaired with obesity - PubMed (original) (raw)
Ciliary neurotrophic factor stimulates muscle glucose uptake by a PI3-kinase-dependent pathway that is impaired with obesity
Gregory R Steinberg et al. Diabetes. 2009 Apr.
Abstract
Objective: Ciliary neurotrophic factor (CNTF) reverses muscle insulin resistance by increasing fatty acid oxidation through gp130-LIF receptor signaling to the AMP-activated protein kinase (AMPK). CNTF also increases Akt signaling in neurons and adipocytes. Because both Akt and AMPK regulate glucose uptake, we investigated muscle glucose uptake in response to CNTF signaling in lean and obese mice.
Research design and methods: Mice were injected intraperitoneally with saline or CNTF, and blood glucose was monitored. The effects of CNTF on skeletal muscle glucose uptake and AMPK/Akt signaling were investigated in incubated soleus and extensor digitorum longus (EDL) muscles from muscle-specific AMPKalpha2 kinase-dead, gp130(DeltaSTAT), and lean and obese ob/ob and high-fat-fed mice. The effect of C2-ceramide on glucose uptake and gp130 signaling was also examined.
Results: CNTF reduced blood glucose and increased glucose uptake in isolated muscles in a time- and dose-dependent manner with maximal effects after 30 min with 100 ng/ml. CNTF increased Akt-S473 phosphorylation in soleus and EDL; however, AMPK-T172 phosphorylation was only increased in soleus. Incubation of muscles from AMPK kinase dead (KD) and wild-type littermates with the PI3-kinase inhibitor LY-294002 demonstrated that PI3-kinase, but not AMPK, was essential for CNTF-stimulated glucose uptake. CNTF-stimulated glucose uptake and Akt phosphorylation were substantially reduced in obesity (high-fat diet and ob/ob) despite normal induction of gp130/AMPK signaling--effects also observed when treating myotubes with C2-ceramide.
Conclusions: CNTF acutely increases muscle glucose uptake by a mechanism involving the PI3-kinase/Akt pathway that does not require AMPK. CNTF-stimulated glucose uptake is impaired in obesity-induced insulin resistance and by ceramide.
Figures
FIG. 1.
In vivo effects of CNTF on blood glucose. A: CNTF tolerance test. Change in blood glucose over time. B: AUC. Animals were injected with either CNTF (0.3 mg/kg) or saline, and changes in tail blood glucose were monitored over 150 min. n = 10. Data are means ± SEM. *Significantly different from saline.
FIG. 2.
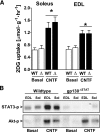
Dose-dependent effect of CNTF on 2-deoxyglucose (2DG) glucose uptake and additive effects of CNTF on AICAR and insulin stimulated glucose uptake. A and B: Muscles were either incubated with vehicle (BAS) condition or stimulated with CNTF at indicated concentrations for 30 min; n = 8. *Significantly different from basal condition (P < 0.05). C and D: Muscles were incubated with CNTF (100 ng/ml), insulin (30 nmol/l), AICAR (2 mmol/l), or indicated combinations, and changes in 2DG uptake are expressed as fold increases over basal. n = 7–8. Data are means ± SEM. *Significantly different from CNTF in the same muscle type.
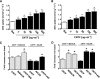
FIG. 3.
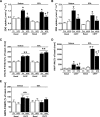
Time-dependent effect of CNTF on 2-deoxyglucose (2DG) uptake, Akt S473, αAMPK T172, and ACCβ S218 phosphorylation in incubated mouse muscle. A: 2DG uptake in incubated soleus and EDL at basal conditions and after incubation with 100 ng/ml CNTF at indicated periods of time. B: Akt S473 phosphorylation in lysates from incubated soleus and EDL at basal conditions and after incubation with 100 ng/ml CNTF at indicated periods of time. C: αAMPK T172 phosphorylation in lysates from incubated soleus and EDL at basal conditions and after incubation with 100 ng/ml CNTF. D: ACCβ S218 phosphorylation in lysates from incubated soleus and EDL at basal conditions and after incubation with 100 ng/ml CNTF. n = 8. Data are means ± SEM. *Significantly different from basal condition.
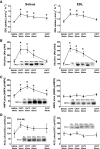
FIG. 4.
Effect of impaired AMPK signaling and the PI3-kinase inhibitor LY-294002 on CNTF-stimulated 2-deoxyglucose (2DG) uptake, AMPK activity, and Akt S473 and STAT3 Y705 phosphorylation in incubated mouse muscle from wild-type (WT) and AMPK KD (Tg) mice. A: 2DG uptake at basal and after incubation with 100 ng/ml CNTF with or without 60 nmol/l of LY-294002 in soleus (left panel) and EDL (right panel) muscle. In addition, incubation of wild-type muscle at basal with 2.8 nmol/l insulin (ins) with and without LY-294002. B: α-Isoform–specific AMPK activity in lysates from incubated soleus (left panel) and EDL (right panel) muscle at basal and after incubation with 100 ng/ml CNTF for 30 min. C: STAT3 Y705 phosphorylation in lysates from incubated EDL muscle at basal and after incubation with 100 ng/ml CNTF with and without LY-294002. D: Akt phosphorylation in lysates from incubated EDL at basal and after incubation with 100 ng/ml CNTF with and without LY-294002. n = 8. Data are means ± SEM. *Significantly different from basal condition. †Significantly different from CNTF or insulin stimulated. Significantly different from WT.
FIG. 5.
CNTF-induced 2-deoxyglucose (2DG) uptake (A) and STAT3 Y705 and Akt S473 phosphorylation (B) in incubated soleus and EDL mouse muscle from wild-type (WT) and gp130ΔSTAT mice. (Muscles were either incubated at basal condition or with 100 ng/ml CNTF for 30 min [n = 8].) Data are means ± SEM. *Significantly different from basal condition.
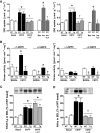
FIG. 6.
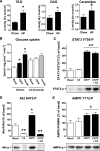
CNTF-stimulated 2-deoxyglucose (2DG) uptake in high-fat diet–fed (A) and ob/ob mice (B), STAT3 Y705 (C), Akt S473 (D), and AMPK T172 (E) phosphorylation in soleus and EDL mouse muscle from mice fed standard chow (Ch) or an HFD for 12 weeks; n = 8–10 for HFD experiment and 7–8 for ob/ob experiment. Data are means ± SEM. *Significantly different from basal condition (P < 0.05, P < 0.001). †Significantly different from CNTF-stimulated chow-fed/control mice.
FIG. 7.
Muscle lipids in gastrocnemius muscle from mice fed standard chow or an HFD and STAT3 Y705, Akt S473, and AMPK T172 phosphorylation in C2C12 myocytes. A: Muscle content of triacylglycerol (TAG), diacylglycerol (DAG), and ceramides. B: CNTF and insulin-stimulated 2-deoxy-
d
-glucose uptake in L6 myotubes treated with either C2-ceramide or vehicle for 6 h. C–E: Phosphorylation of CNTF-activated signaling molecules in C2C12 myotubes treated with C2-ceramide for 6 h or CNTF for 30 min or in combination. *Significantly different from basal condition (P < 0.05, P < 0.001).
Similar articles
- Leukemia inhibitory factor increases glucose uptake in mouse skeletal muscle.
Brandt N, O'Neill HM, Kleinert M, Schjerling P, Vernet E, Steinberg GR, Richter EA, Jørgensen SB. Brandt N, et al. Am J Physiol Endocrinol Metab. 2015 Jul 15;309(2):E142-53. doi: 10.1152/ajpendo.00313.2014. Epub 2015 May 12. Am J Physiol Endocrinol Metab. 2015. PMID: 25968579 - Reduced AMP-activated protein kinase activity in mouse skeletal muscle does not exacerbate the development of insulin resistance with obesity.
Beck Jørgensen S, O'Neill HM, Hewitt K, Kemp BE, Steinberg GR. Beck Jørgensen S, et al. Diabetologia. 2009 Nov;52(11):2395-404. doi: 10.1007/s00125-009-1483-8. Epub 2009 Aug 18. Diabetologia. 2009. PMID: 19688337 - A-769662 activates AMPK beta1-containing complexes but induces glucose uptake through a PI3-kinase-dependent pathway in mouse skeletal muscle.
Treebak JT, Birk JB, Hansen BF, Olsen GS, Wojtaszewski JF. Treebak JT, et al. Am J Physiol Cell Physiol. 2009 Oct;297(4):C1041-52. doi: 10.1152/ajpcell.00051.2009. Epub 2009 Aug 5. Am J Physiol Cell Physiol. 2009. PMID: 19657063 - The role of endothelial insulin signaling in the regulation of glucose metabolism.
Kubota T, Kubota N, Kadowaki T. Kubota T, et al. Rev Endocr Metab Disord. 2013 Jun;14(2):207-16. doi: 10.1007/s11154-013-9242-z. Rev Endocr Metab Disord. 2013. PMID: 23589150 Review. - CNTF: a target therapeutic for obesity-related metabolic disease?
Matthews VB, Febbraio MA. Matthews VB, et al. J Mol Med (Berl). 2008 Apr;86(4):353-61. doi: 10.1007/s00109-007-0286-y. Epub 2008 Jan 22. J Mol Med (Berl). 2008. PMID: 18210031 Review.
Cited by
- Modulation of matrix metalloproteases by ciliary neurotrophic factor in human placental development.
Tossetta G, Fantone S, Busilacchi EM, Di Simone N, Giannubilo SR, Scambia G, Giordano A, Marzioni D. Tossetta G, et al. Cell Tissue Res. 2022 Oct;390(1):113-129. doi: 10.1007/s00441-022-03658-1. Epub 2022 Jul 7. Cell Tissue Res. 2022. PMID: 35794391 Free PMC article. - Biological Effects of Ciliary Neurotrophic Factor on hMADS Adipocytes.
Perugini J, Di Mercurio E, Tossetta G, Severi I, Monaco F, Reguzzoni M, Tomasetti M, Dani C, Cinti S, Giordano A. Perugini J, et al. Front Endocrinol (Lausanne). 2019 Nov 12;10:768. doi: 10.3389/fendo.2019.00768. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31781039 Free PMC article. - AMPK: a cellular metabolic and redox sensor. A minireview.
Shirwany NA, Zou MH. Shirwany NA, et al. Front Biosci (Landmark Ed). 2014 Jan 1;19(3):447-74. doi: 10.2741/4218. Front Biosci (Landmark Ed). 2014. PMID: 24389195 Free PMC article. Review. - AMPK regulates lipid accumulation in skeletal muscle cells through FTO-dependent demethylation of N6-methyladenosine.
Wu W, Feng J, Jiang D, Zhou X, Jiang Q, Cai M, Wang X, Shan T, Wang Y. Wu W, et al. Sci Rep. 2017 Feb 8;7:41606. doi: 10.1038/srep41606. Sci Rep. 2017. PMID: 28176824 Free PMC article. - Ciliary neurotrophic factor protects mice against streptozotocin-induced type 1 diabetes through SOCS3: the role of STAT1/STAT3 ratio in β-cell death.
Rezende LF, Santos GJ, Carneiro EM, Boschero AC. Rezende LF, et al. J Biol Chem. 2012 Dec 7;287(50):41628-39. doi: 10.1074/jbc.M112.358788. Epub 2012 Oct 4. J Biol Chem. 2012. PMID: 23038263 Free PMC article.
References
- Rose AJ, Richter EA: Skeletal muscle glucose uptake during exercise: how is it regulated? Physiology (Bethesda) 20: 260– 270, 2005 - PubMed
- Bruss MD, Arias EB, Lienhard GE, Cartee GD: Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes 54: 41– 50, 2005 - PubMed
- Treebak JT, Glund S, Deshmukh A, Klein DK, Long YC, Jensen TE, Jorgensen SB, Viollet B, Andersson L, Neumann D, Wallimann T, Richter EA, Chibalin AV, Zierath JR, Wojtaszewski JF: AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes 55: 2051– 2058, 2006 - PubMed
- Friedman JE, Dudek RW, Whitehead DS, Downes DL, Frisell WR, Caro JF, Dohm GL: Immunolocalization of glucose transporter GLUT4 within human skeletal muscle. Diabetes 40: 150– 154, 1991 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous