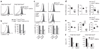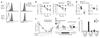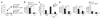Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor - PubMed (original) (raw)
Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor
Yann M Kerdiles et al. Nat Immunol. 2009 Feb.
Abstract
Foxo transcription factors have a conserved role in the adaptation of cells and organisms to nutrient and growth factor availability. Here we show that Foxo1 has a crucial, nonredundant role in T cells. In naive T cells, Foxo1 controlled the expression of the adhesion molecule L-selectin, the chemokine receptor CCR7 and the transcription factor Klf2, and its deletion was sufficient to alter lymphocyte trafficking. Furthermore, Foxo1 deficiency resulted in a severe defect in interleukin 7 receptor alpha-chain (IL-7Ralpha) expression associated with its ability to bind an Il7r enhancer. Finally, growth factor withdrawal induced a Foxo1-dependent increase in Sell, Klf2 and Il7r expression. These data suggest that Foxo1 regulates the homeostasis and life span of naive T cells by sensing growth factor availability and regulating homing and survival signals.
Figures
Figure 1
Foxo1 is preferentially expressed in lymphoid cells. (a) QPCR analysis of Foxo1 mRNA expression in tissues from C57BL/6 mice. LN, lymph node; BM, bone marrow. (b) QPCR analysis of Foxo1 mRNA expression in purified cell subsets from C57BL/6 mice lymph nodes and spleen. The abundance of Foxo1 mRNA in each sample was normalized to that of Hprt1 mRNA and then normalized to the amount obtained for the spleen (set to 1). Data in a,b are mean ± s.d. of duplicate samples. Results are representative of two independent experiments.
Figure 2
Foxo1 is required for maintenance of T cell homeostasis. (a,b) CD44 expression by lymph node TCRβ+ CD4+ and CD8+ gated cells (a) and corresponding cell counts (b; mean ± s.e.m.) of 8- to 12-week-old mice. LN, lymph node. ### and ***, P < 0.0001 wild-type versus knockout for CD44hi and CD44lo populations, respectively). Data represent n = 16 littermate controls and n = 13 Cd4Cre mice analyzed in four independent experiments. (c) CD44 expression by lymph node Vβ5+ cells and (d) total counts of Vβ5+CD4+ cells of 8-week-old mice analyzed in two (spleen) or one (lymph node) experiments. Each circle indicates one mouse (***, P < 0.001).
Figure 3
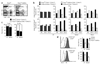
Foxo1 is dispensable for T cell development. (a) Total numbers of thymocytes. Each dot indicates one mouse. (b) Expression of CD4 and CD8 on thymocytes of the indicated genotypes. (c) Left, expression of TCRβ and CD24 on thymocytes of the indicated genotypes. Right, percentages of CD4+ and CD8+ single-positive cells within the population of mature TCRβhiCD24lo thymocytes (mean ± s.e.m.) from 8-week-old mice. Data in a–c represent n = 17 littermate controls and n = 14 Cd4Cre mice, analyzed in five independent experiments (**, P < 0.01). (d) CD4 and CD8 expression on thymocytes from OT-II and OT-I transgenic mice. Data represent three to eight mice per genotype analyzed in two or three independent experiments.
Figure 4
Foxo1 regulates L-selectin, CCR7 and Klf2 expression and T cell homing in vivo. (a) L-selectin expression on CD44loTCRβ+ CD4+ and CD8+ cells of 8- to 12-week-old mice. Results are representative of n = 14 littermate controls and n = 11 Cd4Cre mice analyzed in four independent experiments. (b) Quantification of L-selectin expression on Vβ5+CD4+ cells of 8-week-old mice (mean ± s.e.m.). LN, lymph node. Data represent n = 4 littermate controls and n = 7 Cd4Cre mice analyzed in two independent experiments (***, P < 0.0001). MFI, mean fluorescence intensity. (c–e) _Foxo1_f/f-ERCre mice and littermate controls (CD45.2+) were treated for 5 d with tamoxifen and rested for 5 d. (c) Quantification of L-selectin and CCR7 expression on lymph node CD44loTCRβ+ CD4+ and CD8+ cells (mean ± s.e.m.). Data represent n ≥ 4 mice per genotype, analyzed in two to three independent experiments (***, P < 0.0001). (d) QPCR analysis of Sell, Ccr7 and Klf2 mRNA expression, normalized to Hprt1 mRNA, in purified lymph node T cells. Each circle indicates one mouse (***, P < 0.0001). (e) lymph node T cells were purified, and one of two populations was labeled with CFSE. The two populations were mixed at a 1:1 ratio and injected into C57BL/6 CD45.1+ mice (10 × 106 cells per mouse). Donor cell recovery was analyzed 18 h later in peripheral blood, lymph nodes and spleen (CD45.2+-gated CFSE+ versus CFSE− cells). Each circle indicates one host mouse. Results are from two independent experiments (***, P < 0.0001). (f) Number of naive T cells (mean ± s.e.m.) in 3-week-old mice. Data represent n = 6 littermate controls and n = 9 Cd4Cre mice analyzed in two independent experiments (***, P < 0.0001).
Figure 5
Foxo1 is required for naive T cell survival. (a) CD4+ and CD8+ cells in peripheral blood of 8- to 12-week-old mice, as assessed by flow cytometry. Results are representative of n = 5 littermate controls and n = 9 Cd4Cre mice analyzed in two independent experiments. (b) Purified lymph node T cells were mixed at a 1:1 ratio based on the number of CD8+CD44lo cells and transferred into C57BL/6 CD45.1+ hosts (2 × 106 total CD8+CD44lo cells). Donor cell recovery was analyzed before injection (Mix.) and at the indicated time points (CD45.2+-gated CD45.1+ versus CD45.1− cells). LN, lymph node. Representative results from two independent experiments with n ≥ 3 mice per group and per time point (initial difference in CD4+CD44lo proportion comes from a modification of the CD4:CD8 ratio in _Foxo1_f/f-Cd4Cre mice). (c) T and B cell recovery in mixed bone chimeras 8 weeks after reconstitution (mean ± s.e.m. of n = 3 mice per group). (d) Quantification of Bcl-2 expression in CD44lo T cells from 8- to 12-week-old mice (mean ± s.e.m.). Data represent n = 9 littermate controls and n = 12 Cd4Cre mice analyzed in two independent experiments (***, P < 0.0001).
Figure 6
Foxo1 is required for IL-7Rα expression in naive T cells and binds to an Il7r enhancer. (a) CD127 and CD132 expression on CD44lo T cells from 8- to 12-week-old mice. Data represent n = 9 littermate controls (LT) and n = 10 Cd4Cre mice (KO) analyzed in three independent experiments. (b) Lymph node (LN) cells were cultured in medium supplemented or not with IL-7 for 3 d, and the proportion of live (annexin V–negative, AnnV−) CD44lo CD4+ and CD8+ T cells was measured by flow cytometry at the indicated time points (mean ± s.d. of triplicate cultures). Results are representative of three independent experiments. (c) Quantification of CD127 expression on CD44lo CD4+ and CD8+ T cells after tamoxifen treatment in _Foxo1_f/f-ERCre mice (mean ± s.e.m.). MFI, mean fluorescence intensity. Results are representative of three independent experiments with n = 7–11 mice per time point and per genotype. (d) QPCR analysis of Il7r and Il2rg mRNA expression in purified lymph node T cells on day 11 after the beginning of tamoxifen treatment. Each dot indicates one mouse (***, P < 0.0001). (e) Il7r locus. (f) Chromatin immunoprecipitation analysis of Foxo1 binding to the Il7r locus in purified lymph node T cells from littermate controls and Cd4Cre mice. Results are relative to the value obtained for the control immunoprecipitation (Ig), with Foxo1-sufficient T cells set as 1 (mean ± s.d. of duplicate samples). ND, not detected. Results are representative of four independent experiments.
Figure 7
Foxo1-mediated control of Il-7Rα and trafficking receptors after cell starvation. (a) CD127 expression on lymph node T cells freshly isolated and rested overnight (ON) in medium (mean ± s.d. of triplicate cultures). (b–d) _Foxo1_f/f-ERCre mice and littermate controls were treated with tamoxifen for 5 d and rested for 3 d. Lymph node T cells were then purified and cultured overnight in medium supplemented with IL-7 (10 ng/ml) as indicated. (b) QPCR analysis of Il7r mRNA, normalized to Hprt mRNA, after overnight culture. c, Chromatin immunoprecipitation analysis of Foxo1 binding to IL7r ECR2. (d) QPCR analysis of Sell, Klf2 and Ccr7 mRNA. Results are presented as fold change (mean ± s.d. of triplicate cultures) relative to the value obtained for freshly isolated T cells set to 1. Results are representative of two (a,c) or three (b,d) independent experiments (**, P < 0.01; ***, P < 0.0001).
Comment in
- Homeostasis of naive T cells: the Foxo that fixes.
Freitas AA, Rocha B. Freitas AA, et al. Nat Immunol. 2009 Feb;10(2):133-4. doi: 10.1038/ni0209-133. Nat Immunol. 2009. PMID: 19148194 No abstract available.
Similar articles
- Suppression of Foxo1 activity and down-modulation of CD62L (L-selectin) in HIV-1 infected resting CD4 T cells.
Trinité B, Chan CN, Lee CS, Mahajan S, Luo Y, Muesing MA, Folkvord JM, Pham M, Connick E, Levy DN. Trinité B, et al. PLoS One. 2014 Oct 16;9(10):e110719. doi: 10.1371/journal.pone.0110719. eCollection 2014. PLoS One. 2014. PMID: 25330112 Free PMC article. - FOXO1 regulates L-Selectin and a network of human T cell homing molecules downstream of phosphatidylinositol 3-kinase.
Fabre S, Carrette F, Chen J, Lang V, Semichon M, Denoyelle C, Lazar V, Cagnard N, Dubart-Kupperschmitt A, Mangeney M, Fruman DA, Bismuth G. Fabre S, et al. J Immunol. 2008 Sep 1;181(5):2980-9. doi: 10.4049/jimmunol.181.5.2980. J Immunol. 2008. PMID: 18713968 - T cells require Foxo1 to populate the peripheral lymphoid organs.
Gubbels Bupp MR, Edwards B, Guo C, Wei D, Chen G, Wong B, Masteller E, Peng SL. Gubbels Bupp MR, et al. Eur J Immunol. 2009 Nov;39(11):2991-9. doi: 10.1002/eji.200939427. Eur J Immunol. 2009. PMID: 19658095 Free PMC article. - FOXO1 transcription factor: a critical effector of the PI3K-AKT axis in B-cell development.
Szydłowski M, Jabłońska E, Juszczyński P. Szydłowski M, et al. Int Rev Immunol. 2014 Mar;33(2):146-57. doi: 10.3109/08830185.2014.885022. Epub 2014 Feb 20. Int Rev Immunol. 2014. PMID: 24552152 Review. - The Role of Forkhead Box 1 (FOXO1) in the Immune System: Dendritic Cells, T Cells, B Cells, and Hematopoietic Stem Cells.
Cabrera-Ortega AA, Feinberg D, Liang Y, Rossa C Jr, Graves DT. Cabrera-Ortega AA, et al. Crit Rev Immunol. 2017;37(1):1-13. doi: 10.1615/CritRevImmunol.2017019636. Crit Rev Immunol. 2017. PMID: 29431075 Free PMC article. Review.
Cited by
- T follicular helper cells in space-time.
Qi H. Qi H. Nat Rev Immunol. 2016 Oct;16(10):612-25. doi: 10.1038/nri.2016.94. Epub 2016 Aug 30. Nat Rev Immunol. 2016. PMID: 27573485 Review. - The transcription factor KLF2 restrains CD4⁺ T follicular helper cell differentiation.
Lee JY, Skon CN, Lee YJ, Oh S, Taylor JJ, Malhotra D, Jenkins MK, Rosenfeld MG, Hogquist KA, Jameson SC. Lee JY, et al. Immunity. 2015 Feb 17;42(2):252-264. doi: 10.1016/j.immuni.2015.01.013. Immunity. 2015. PMID: 25692701 Free PMC article. - Role of PI3K/Akt signaling in memory CD8 T cell differentiation.
Kim EH, Suresh M. Kim EH, et al. Front Immunol. 2013 Feb 1;4:20. doi: 10.3389/fimmu.2013.00020. eCollection 2013. Front Immunol. 2013. PMID: 23378844 Free PMC article. - CCR7/CCL19 controls expression of EDG-1 in T cells.
Shannon LA, McBurney TM, Wells MA, Roth ME, Calloway PA, Bill CA, Islam S, Vines CM. Shannon LA, et al. J Biol Chem. 2012 Apr 6;287(15):11656-64. doi: 10.1074/jbc.M111.310045. Epub 2012 Feb 13. J Biol Chem. 2012. PMID: 22334704 Free PMC article. - Akt2 Regulates the Differentiation and Function of NKT17 Cells via FoxO-1-ICOS Axis.
Niu L, Xuan X, Wang J, Li L, Yang D, Jing Y, Westerberg LS, Liu C. Niu L, et al. Front Immunol. 2018 Sep 12;9:1940. doi: 10.3389/fimmu.2018.01940. eCollection 2018. Front Immunol. 2018. PMID: 30258434 Free PMC article. Retracted.
References
- Marrack P, Kappler J. Control of T cell viability. Annu. Rev. Immunol. 2004;22:765–787. - PubMed
- Almeida AR, Rocha B, Freitas AA, Tanchot C. Homeostasis of T cell numbers: from thymus production to peripheral compartmentalization and the indexation of regulatory T cells. Semin. Immunol. 2005;17:239–249. - PubMed
- Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. - PubMed
- van der Vos KE, Coffer PJ. FOXO-binding partners: it takes two to tango. Oncogene. 2008;27:2289–2299. - PubMed
- Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27:2276–2288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K26 RR024196/RR/NCRR NIH HHS/United States
- K26 RR024196-02/RR/NCRR NIH HHS/United States
- R01 AI073885/AI/NIAID NIH HHS/United States
- R01 AI073885-01A2/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous