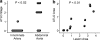Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE-/- mice - PubMed (original) (raw)
. 2009 Jan 16;206(1):233-48.
doi: 10.1084/jem.20080752. Epub 2009 Jan 12.
Katharina Lötzer, Sandra Döpping, Markus Hildner, Dörte Radke, Michael Beer, Rainer Spanbroek, Beatrix Lippert, Catherine A Reardon, Godfrey S Getz, Yang-Xin Fu, Thomas Hehlgans, Reina E Mebius, Michael van der Wall, Dagmar Kruspe, Christoph Englert, Agnes Lovas, Desheng Hu, Gwendalyn J Randolph, Falk Weih, Andreas J R Habenicht
Affiliations
- PMID: 19139167
- PMCID: PMC2626665
- DOI: 10.1084/jem.20080752
Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE-/- mice
Rolf Gräbner et al. J Exp Med. 2009.
Abstract
Atherosclerosis involves a macrophage-rich inflammation in the aortic intima. It is increasingly recognized that this intimal inflammation is paralleled over time by a distinct inflammatory reaction in adjacent adventitia. Though cross talk between the coordinated inflammatory foci in the intima and the adventitia seems implicit, the mechanism(s) underlying their communication is unclear. Here, using detailed imaging analysis, microarray analyses, laser-capture microdissection, adoptive lymphocyte transfers, and functional blocking studies, we undertook to identify this mechanism. We show that in aged apoE(-/-) mice, medial smooth muscle cells (SMCs) beneath intimal plaques in abdominal aortae become activated through lymphotoxin beta receptor (LTbetaR) to express the lymphorganogenic chemokines CXCL13 and CCL21. These signals in turn trigger the development of elaborate bona fide adventitial aortic tertiary lymphoid organs (ATLOs) containing functional conduit meshworks, germinal centers within B cell follicles, clusters of plasma cells, high endothelial venules (HEVs) in T cell areas, and a high proportion of T regulatory cells. Treatment of apoE(-/-) mice with LTbetaR-Ig to interrupt LTbetaR signaling in SMCs strongly reduced HEV abundance, CXCL13, and CCL21 expression, and disrupted the structure and maintenance of ATLOs. Thus, the LTbetaR pathway has a major role in shaping the immunological characteristics and overall integrity of the arterial wall.
Figures
Figure 1.
Incidence of ATLOs in different aorta segments and association of ATLOs and plaques. (a) Preferential occurrence of ATLOs in the abdominal aorta; innominate arteries and abdominal aortae were examined for ATLO stages in aged _apoE_−/− mice. chi-Square (χ2) test, P < 0.02. (b) ATLO sizes correlate with plaque sizes; morphometry was performed by determination of plaque size/media size or ATLO size/media size (n = 11 mice). Pearson correlation coefficient: 0.783. P < 0.01.
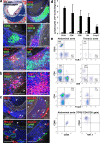
Figure 2.
ATLO cellularity and structure and quantification of leukocyte subsets in abdominal and thoracic aorta segments. (a) ATLO position relative to media (dashed lines) and plaque (P) of aged _apoE_−/− mouse (Oil Red O/hematoxylin); B cell follicle (B220); and T cell area (CD3ε). FDCs in GC (CD35, filled arrow), plasma cells (CD138, open arrow), and DAPI (DNA); GCs contain Ki67+ (triangles) cells in PNA+ areas (asterisks) and follicular mantle B cells (IgD+; open triangles). (b) Foxp3+ T reg cells in ATLO T cell area (left, arrows) and in T cell area of paraaortic LN (right). (c) HEVs (PNAd; top left, filled arrows), blood vessels (MECA-32; top and bottom right; filled arrows indicate HEVs, open arrows indicate blood vessels), ATLO lymph vessel (Lyve1; top right and bottom left, open triangles), and normal lymph vessels (top right, filled triangle); asterisk indicates vena cava. Bars: 100 μm. (d) Absolute numbers of lymphocyte subsets per aorta segment (open columns, thoracic aorta; shaded columns, abdominal aorta). CD19, P < 0.0005; CD4, P < 0.05; CD8, n.s.; DN, P < 0.005; Foxp3, P < 0.02; one-sided unpaired Student's t test. (e) Flow cytometric analysis of aortae from old apoE−/− mice. Aortae were separated into similarly sized abdominal and thoracic segments, and single-cell suspensions were analyzed for expression of CD19/TCRβ (top two plots, lymphocyte gate within the CD45+ population), as well as CD4/CD8 and CD4/Foxp3 (middle four plots, TCRβ+ gate within CD45+ lymphocytes). TCRβ+ CD4−CD8− DN T cells from the abdominal aorta were further characterized for CD69/CD28 and CD44/NK1.1 expression (bottom two plots, TCRβ+CD4−CD8− gate). DN T cells were also CD25+Foxp3− (not depicted). Numbers in quadrants represent percentages of positive cells (mean values ± SD; n = 7–12 mice).
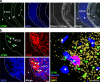
Figure 3.
ATLOs promote lymphocyte recruitment and recirculation into the arterial wall. CFSE labeling and i.v. application of splenocytes was performed as described in Materials and methods. 18 h later, aorta was prepared and consecutive sections were examined. (a) Overview of abdominal aorta segment with adjacent intima lesion and ATLO at 18 h reveals selective presence of CFSE+ splenocytes in ATLO, but none in adjacent adventitia, media, or plaque (filled arrow). Unspecific autofluorescence was subtracted from the specific CFSE-related fluorescence as described in Materials and methods. L, lumen; A, adventitia; M, media; P, plaque. (b) CFSE+ splenocytes preferentially accumulate in ATLO T cell area (left images, filled arrows) when compared with B cell follicles (open diamonds); CFSE+ cells are associated with HEVs (MECA-32+PNAd+ structure, open arrows), but not with blood vessel lumina (MECA-32+PNAd− structures, asterisks; Video 1, available at
http://www.jem.org/cgi/content/full/jem.20080752/DC1
). Bars, 20 μm.
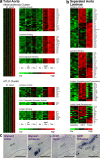
Figure 4.
Lifespan aorta gene expression profiling identifies candidate atherosclerosis versus ATLO genes. Microarrays were prepared from total aortae of individual mice of each genotype (total aorta in a) or from LCM-derived tissues (separated aorta laminae in b). Differentially regulated probe sets were identified as described in Materials and methods. Probe sets specifying genes with the highest signal strengths are displayed. (a) Atherosclerosis cluster displays genes up-regulated between 6 and 32 wk; heat maps at right indicate significantly up-regulated genes (unpaired Student's t test; P < 0.05) of GO terms cytokine activity, cytokine binding, and immunoglobulin binding. ATLO cluster displays genes up-regulated between 32 and 78 wk; heat maps at right indicate significantly up-regulated genes of GO terms cytokine activity, CXC chemokine receptor activity, and antigen binding, (b) Heat maps display LCM preparations of adventitia without plaque or ATLO (left; n = 4 mice), ATLOs (adventitia adjacent to intima plaque; middle; n = 4 mice), or intima plaques (right; n = 3 mice). GO terms for cytokine activity, cytokine binding, antigen processing and presentation, and LN development were examined. (c) Glycam1 and Ccl21 mRNA in situ hybridization analyses of ATLO-burdened abdominal aorta segments. Sense and antisense cRNA probes were generated, and in situ hybridization analyses were performed (filled triangle, HEV; asterisk, lymph vessel). Media and lesions did not show specific Glycam1 or Ccl21 mRNA signals. Broken lines designate media. Bars, 100 μm.
Figure 5.
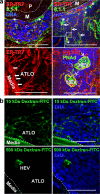
ATLO conduits connect media SMCs with ATLO compartments and facilitate transport of small molecular weight molecules. (a) Conduits connect the media (M) with HEVs. ECM meshwork (ER-TR7; top left, open arrows) associated with (top left, asterisk) and enfolded by 8.1.1+ fibroblastic reticular cells (top right, filled arrows). Conduit pseudolumen (inset, top right). Conduits at the ATLO periphery (ER-TR7) connect the media with HEVs (bottom panels, arrow; Fig. S4 and Video 2; Fig. S5 and Video 3). (b) ATLO conduits transport low molecular weight molecules. 10 or 500 kD fluorescent dextran was injected i.v.; 15 min later, ATLOs were prepared as described in Materials and methods. Dotted lines indicate ATLO/media border. Note presence of 500 kD dextran in HEV (bottom right) and presence of 10 kD, but not 500 kD, dextran in media. Bars, 50 μm.
Figure 6.
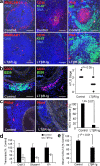
Media SMCs sandwiched between lesions and ATLOs are activated and express the lymphorganogenic chemokines CXCL13 and CCL21. (a) CXCL13 and CCL21 of ATLO-burdened aorta segment (top). Media SMCs and cells with DC morphology stain positive for CXCL13 in B cell follicle (top left, open triangles) and for CCL21 in T cell area (top right, filled arrows), whereas SMCs of aorta segments without adjacent ATLO (thoracic aorta with plaque) do not (bottom). Medium-sized ATLO blood vessels stain positive for CCL21 (top right, filled arrows), whereas small blood vessels (top right, open arrows) in B cell follicles are CCL21−. L, lymph vessel; P, plaque. (b) Colocalization of SMA and CXCL13 and SMA and CCL21 with VCAM-1 in abdominal aorta segments adjacent to ATLO. Bars, 100 μm.
Figure 7.
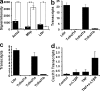
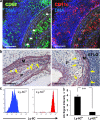
SMCs express LTβR and TNFRSF1A and induce CXCL13 mRNA. (a) Microarray analyses of LCM-derived media (shaded columns) versus adventitia (open columns) from 78-wk-old wild-type mice were examined for signal strengths of Acta2, Mbp, and Ltbr. Means of three independent LCM preparations of individual aortae ± SEM. *, P < 0.05; **, P < 0.001; two-sided, unpaired Student's t test. (b) qRT-PCR analysis of the expression of Ltbr, Tnfrsf14, Tnfrsf1a, and Tnfrsf1b expression in freshly isolated SMCs. Means of three independent SMC cultures ± SEM. (c) Same analyses as in b in cultured SMCs. (d) Cultured SMCs were stimulated with 10 μg/ml α-LTβR, 1 ng/ml TNF, or both for 24 h, and Cxcl13 transcripts were determined by qRT-PCR. Data represent means of five independent experiments ± SEM. P < 0.05, one-sided paired Student's t test.
Figure 8.
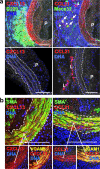
In vivo blockade of LTβR by soluble LTβR-Ig disrupts ATLO structure, reduces Cxcl13 and Glycam1 mRNAs, and attenuates serum CXCL13 levels. _ApoE_−/− mice at 75 wk were treated three times with 75 μg LTβR-Ig (n = 11) or the same concentration of human IgG (n = 6) i.p. every 7 d for 3 consecutive weeks. (a) At the end of the treatment period, LTβR-Ig treatment was monitored in the spleen by loss of integrity of marginal zones (MAdCAM-1), FDCs (CD35), cell proliferation (Ki67), centrocytes (PNA), and follicular mantle B cells (IgD); note loss of FDCs and centrocytes and that mantle IgD+ B cells are less affected. (b) ATLO stages were determined after staining with CD3ε (T cells), B220 (B cells), and CD35 antisera (FDC). χ2 test, P < 0.05. (c) HEVs (PNAd+) abundance was determined by the χ2 test in LTβR-Ig versus control IgG-treated mice (P < 0.05); representative stainings are shown at left. (d) Aortae of LTβR-Ig– (n = 6) or control IgG-treated (n = 3) mice were examined by qRT-PCR for Cxcl13 (P < 0.01), Glycam1 (P < 0.05), and Il7r (P < 0.05) mRNAs; unpaired Student's t test; means ± SEM. (e) serum CXCL13 levels of control hu-IgG–treated mice (n = 4) or LTβR-Ig–treated mice (n = 7). Means ± SEM (P < 0.01). Bars, 100 μm.
Figure 9.
Thoracic and abdominal aorta media of aged _apoE_−/− aorta is infiltrated by leukocytes and shows differential loss of integrity. (a) ATLO staining for CD68+ macrophages (left, filled arrows) and CD11c+ macrophages/DCs (right, open arrows) show leukocyte infiltration of inner and outer media. (b) Elastin staining of ATLO-free diseased abdominal aorta shows loss of elastic fiber integrity in the inner media (left arrows), whereas ATLO-associated abdominal aorta shows loss of integrity in inner and outer media (right arrows). Foam cells adjacent to media are often associated with elastin strand breaks (asterisks). (c) FACS-sorted blood monocyte subset (left) microarrays (right) reveal differential Ltb mRNA expression (two-sided unpaired Student's t test; P < 0.0001). Bars, 100 μm.
Figure 10.
Schematic choreography of cells and molecules in ATLO neogenesis. Blood monocytes/macrophages/foam cells, T cells, and SMCs form atherosclerotic lesions in the intima and initiate a transmural arterial wall inflammation pathway. Lesion cells such as CD11c+ and/or CD11c− myeloid cells and T cells produce TNFR agonists LTβ and TNF and, after LTβR/TNFRSF1A activation and induction of alternative and classical NF-κB signaling pathways, media SMCs are activated and acquire features of lymphoid tissue organizers. Lymphorganogenic CXCL13/CCL21 and other mediators gain access to cells residing in the adventitia, recruit B and T cells through vasa vasora, and promote lymphocyte and stromal cell activation. A tissue microenvironment is then generated that induces conduit neogenesis via stimulation of reticular fibroblasts, DC recruitment, HEV formation, ectopic lymph vessel, and blood vessel neogenesis. ATLO formation accelerates and lymphocyte recirculation is markedly facilitated, B cell follicles form, and T reg cells in T cell areas are recruited. Transmural inflammation also generates autoantigen(s), although their nature, transport routes, and functional roles all remain to be defined. Accelerated B cell recruitment leads to B cell follicle formation, and ectopic GCs are activated. Autoantigen-binding FDCs initiates B cell proliferation, differentiation, and affinity maturation. Memory cells and/or plasma cells, and possibly autoreactive T/B cells, together with T reg cell deficiency may lead to clinically apparent and overt autoimmunity and arterial wall pathology.
Similar articles
- Mouse aorta smooth muscle cells differentiate into lymphoid tissue organizer-like cells on combined tumor necrosis factor receptor-1/lymphotoxin beta-receptor NF-kappaB signaling.
Lötzer K, Döpping S, Connert S, Gräbner R, Spanbroek R, Lemser B, Beer M, Hildner M, Hehlgans T, van der Wall M, Mebius RE, Lovas A, Randolph GJ, Weih F, Habenicht AJ. Lötzer K, et al. Arterioscler Thromb Vasc Biol. 2010 Mar;30(3):395-402. doi: 10.1161/ATVBAHA.109.191395. Epub 2010 Feb 5. Arterioscler Thromb Vasc Biol. 2010. PMID: 20139367 Free PMC article. - Artery Tertiary Lymphoid Organs Control Aorta Immunity and Protect against Atherosclerosis via Vascular Smooth Muscle Cell Lymphotoxin β Receptors.
Hu D, Mohanta SK, Yin C, Peng L, Ma Z, Srikakulapu P, Grassia G, MacRitchie N, Dever G, Gordon P, Burton FL, Ialenti A, Sabir SR, McInnes IB, Brewer JM, Garside P, Weber C, Lehmann T, Teupser D, Habenicht L, Beer M, Grabner R, Maffia P, Weih F, Habenicht AJ. Hu D, et al. Immunity. 2015 Jun 16;42(6):1100-15. doi: 10.1016/j.immuni.2015.05.015. Immunity. 2015. PMID: 26084025 Free PMC article. - M1 macrophages act as LTβR-independent lymphoid tissue inducer cells during atherosclerosis-related lymphoid neogenesis.
Guedj K, Khallou-Laschet J, Clement M, Morvan M, Gaston AT, Fornasa G, Dai J, Gervais-Taurel M, Eberl G, Michel JB, Caligiuri G, Nicoletti A. Guedj K, et al. Cardiovasc Res. 2014 Mar 1;101(3):434-43. doi: 10.1093/cvr/cvt263. Epub 2013 Nov 22. Cardiovasc Res. 2014. PMID: 24272771 - Artery Tertiary Lymphoid Organs: Powerhouses of Atherosclerosis Immunity.
Yin C, Mohanta SK, Srikakulapu P, Weber C, Habenicht AJ. Yin C, et al. Front Immunol. 2016 Oct 10;7:387. doi: 10.3389/fimmu.2016.00387. eCollection 2016. Front Immunol. 2016. PMID: 27777573 Free PMC article. Review. - Artery tertiary lymphoid organs contribute to innate and adaptive immune responses in advanced mouse atherosclerosis.
Mohanta SK, Yin C, Peng L, Srikakulapu P, Bontha V, Hu D, Weih F, Weber C, Gerdes N, Habenicht AJ. Mohanta SK, et al. Circ Res. 2014 May 23;114(11):1772-87. doi: 10.1161/CIRCRESAHA.114.301137. Circ Res. 2014. PMID: 24855201 Review.
Cited by
- Fine-tuning of dendritic cell biology by the TNF superfamily.
Summers deLuca L, Gommerman JL. Summers deLuca L, et al. Nat Rev Immunol. 2012 Apr 10;12(5):339-51. doi: 10.1038/nri3193. Nat Rev Immunol. 2012. PMID: 22487654 Review. - Tertiary Lymphoid Structures in Cancers: Prognostic Value, Regulation, and Manipulation for Therapeutic Intervention.
Sautès-Fridman C, Lawand M, Giraldo NA, Kaplon H, Germain C, Fridman WH, Dieu-Nosjean MC. Sautès-Fridman C, et al. Front Immunol. 2016 Oct 3;7:407. doi: 10.3389/fimmu.2016.00407. eCollection 2016. Front Immunol. 2016. PMID: 27752258 Free PMC article. Review. - Innate immunity of vascular smooth muscle cells contributes to two-wave inflammation in atherosclerosis, twin-peak inflammation in aortic aneurysms and trans-differentiation potential into 25 cell types.
Yang Q, Saaoud F, Lu Y, Pu Y, Xu K, Shao Y, Jiang X, Wu S, Yang L, Tian Y, Liu X, Gillespie A, Luo JJ, Shi XM, Zhao H, Martinez L, Vazquez-Padron R, Wang H, Yang X. Yang Q, et al. Front Immunol. 2024 Jan 24;14:1348238. doi: 10.3389/fimmu.2023.1348238. eCollection 2023. Front Immunol. 2024. PMID: 38327764 Free PMC article. - Cross-reacting antibacterial auto-antibodies are produced within coronary atherosclerotic plaques of acute coronary syndrome patients.
Canducci F, Saita D, Foglieni C, Piscopiello MR, Chiesa R, Colombo A, Cianflone D, Maseri A, Clementi M, Burioni R. Canducci F, et al. PLoS One. 2012;7(8):e42283. doi: 10.1371/journal.pone.0042283. Epub 2012 Aug 6. PLoS One. 2012. PMID: 22879930 Free PMC article. - Persistent B Cell-Derived MHC Class II Signaling Is Required for the Optimal Maintenance of Tissue-Resident Helper T Cells.
Son YM, Cheon IS, Li C, Sun J. Son YM, et al. Immunohorizons. 2024 Feb 1;8(2):163-171. doi: 10.4049/immunohorizons.2300093. Immunohorizons. 2024. PMID: 38345472 Free PMC article.
References
- Glass, C.K., and J.L. Witztum. 2001. Atherosclerosis. The road ahead. Cell. 104:503–516. - PubMed
- Libby, P. 2002. Inflammation in atherosclerosis. Nature. 420:868–874. - PubMed
- Wick, G., M. Knoflach, and Q. Xu. 2004. Autoimmune and inflammatory mechanisms in atherosclerosis. Annu. Rev. Immunol. 22:361–403. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous