Subdivisions of primary motor cortex based on cortico-motoneuronal cells - PubMed (original) (raw)
Subdivisions of primary motor cortex based on cortico-motoneuronal cells
Jean-Alban Rathelot et al. Proc Natl Acad Sci U S A. 2009.
Abstract
We used retrograde transneuronal transport of rabies virus from single muscles of rhesus monkeys to identify cortico-motoneuronal (CM) cells in the primary motor cortex (M1) that make monosynaptic connections with motoneurons innervating shoulder, elbow, and finger muscles. We found that M1 has 2 subdivisions. A rostral region lacks CM cells and represents an "old" M1 that is the standard for many mammals. The descending commands mediated by corticospinal efferents from old M1 must use the integrative mechanisms of the spinal cord to generate motoneuron activity and motor output. In contrast, a caudal region of M1 contains shoulder, elbow, and finger CM cells. This region represents a "new" M1 that is present only in some higher primates and humans. The direct access to motoneurons afforded by CM cells enables the newly recognized M1 to bypass spinal cord mechanisms and sculpt novel patterns of motor output that are essential for highly skilled movements.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
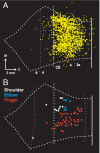
Distribution of CM cells innervating the motoneurons of a shoulder or an elbow muscle. (Upper) Results from injections of rabies into the SpD muscle. (Lower) Results from injections into the lateral head of the lTri muscle. Each map shows an unfolded reconstruction of layer V from an experimental case. Each dot represents a labeled CM cell. In this and other figures, the vertical dashed line in each map represents the edge of the CS, and vertical dotted lines indicate the approximate location of cytoarchitectonic borders. The central Inset shows the general location of the reconstructed area on a lateral view of the macaque cerebral hemisphere. Note that most shoulder and elbow CM cells are located medially in the CS. ArS, arcuate sulcus; C, caudal; M, medial; Gyrus, crest of the precentral gyrus; Sulcus, anterior bank of the CS.
Fig. 2.
Overlap maps of CM cells innervating proximal versus distal muscles. (A) Black dots represent CM cells labeled by injections into SpD (n = 2). (B) Black dots represent CM cells labeled by injections into lTri (n = 2). Gray dots in A and B represent CM cells labeled after virus injections into finger muscles (ADP or ABPL) (from figure 3 in ref. 2). Note that some elbow and shoulder CM cells are found in regions of the CS where there is a high density of finger CM cells.
Fig. 3.
Topographic organization of CM cells in M1. (Upper) Density analysis of CM cells innervating shoulder (Left), elbow (Center), or finger (Right) motoneurons. The color scale at the right indicates the density of labeled neurons as percentages relative to the maximum peak density. Note the presence of a large medial group of shoulder CM cells along with a small lateral group. (Lower) (Left) Density peaks of shoulder (white), elbow (blue), and finger (red) CM cells. The cutoff for shoulder and finger CM cells, upper 75%; the cutoff for elbow CM cells, upper 87.5%. Note that in general the peak densities of shoulder and elbow CM cells are located medial to the peak density of finger CM cells. Even so, there is considerable intermingling of the different populations of CM cells. (Right) Results of intracortical stimulation (redrawn from ref. with permission of the American Physiological Society). Colors indicate the movement evoked by threshold stimulation at each site: shoulder (white), elbow (blue), or finger (red). Symbol size indicates the threshold for each site (key below). In the CS, most shoulder and elbow sites were located medial to finger sites. However, a small number of high threshold shoulder and elbow sites were located more laterally in the CS. Compare the location of these sites with the small lateral group of shoulder CM cells shown in Upper Left.
Fig. 4.
Correspondence between CM cells and low threshold sites. (A) Map of all CM cells (yellow dots) labeled by rabies injections into shoulder (SpD), elbow (lTri), and finger (ABPL, ADP, and EDC) muscles. The map is an overlap of the data presented in Fig. 1 and the data presented in figure 3 in ref. . (B) Plot of sites where intracortical microstimulation at the lowest threshold (2–5 μA) evoked shoulder (white), elbow (blue), and finger (red) movements (data from ref. 3). Note that the lowest threshold sites for evoking movement and CM cells are most concentrated in the caudal portion of M1 in the CS.
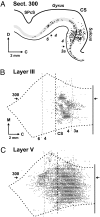
Fig. 5.
Third-order neurons in motor cortex. We injected rabies virus into the ABPL muscle, and the survival time was set to allow labeling of third-order neurons (experiment JA29 in
Table S2
). (A) Plot of a sagittal section (300) through M1 showing labeled neurons (dots). Layer V is shaded gray. (B) Map of labeled neurons in layer III. (C) Map of labeled neurons in layer V. The location of section 300 is indicated on the maps by horizontal arrows. C, caudal; D, dorsal; M, medial; SPcS, superior precentral sulcus. Note that third-order neurons in layer III are largely confined to the caudal portion of M1 in the CS, whereas layer V neurons (second- and third-order) are found in rostral as well as caudal portions of M1.
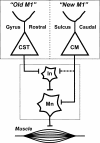
Fig. 6.
New and old M1. New M1 is located caudally in the CS and has CM cells that make direct connections with motoneurons. In contrast, old M1 is located rostrally on the precentral gyrus and lacks CM cells. However, old M1 has CST neurons that influence motoneurons indirectly through their connections with spinal interneurons. CM, cortico-motoneuronal; CST, corticospinal; In, interneurons; Mn, motoneurons.
Similar articles
- Corticospinal Inputs to Primate Motoneurons Innervating the Forelimb from Two Divisions of Primary Motor Cortex and Area 3a.
Witham CL, Fisher KM, Edgley SA, Baker SN. Witham CL, et al. J Neurosci. 2016 Mar 2;36(9):2605-16. doi: 10.1523/JNEUROSCI.4055-15.2016. J Neurosci. 2016. PMID: 26937002 Free PMC article. - Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: an anatomical and electrophysiological study.
Maier MA, Armand J, Kirkwood PA, Yang HW, Davis JN, Lemon RN. Maier MA, et al. Cereb Cortex. 2002 Mar;12(3):281-96. doi: 10.1093/cercor/12.3.281. Cereb Cortex. 2002. PMID: 11839602 - Corticomotoneuronal cells are "functionally tuned".
Griffin DM, Hoffman DS, Strick PL. Griffin DM, et al. Science. 2015 Nov 6;350(6261):667-70. doi: 10.1126/science.aaa8035. Science. 2015. PMID: 26542568 Free PMC article. - The importance of the cortico-motoneuronal system for control of grasp.
Lemon RN, Baker SN, Davis JA, Kirkwood PA, Maier MA, Yang HS. Lemon RN, et al. Novartis Found Symp. 1998;218:202-15; discussion 215-8. doi: 10.1002/9780470515563.ch11. Novartis Found Symp. 1998. PMID: 9949822 Review. - Descending pathways in motor control.
Lemon RN. Lemon RN. Annu Rev Neurosci. 2008;31:195-218. doi: 10.1146/annurev.neuro.31.060407.125547. Annu Rev Neurosci. 2008. PMID: 18558853 Review.
Cited by
- Timing of Cortico-Muscle Transmission During Active Movement.
Van Acker GM 3rd, Luchies CW, Cheney PD. Van Acker GM 3rd, et al. Cereb Cortex. 2016 Aug;26(8):3335-44. doi: 10.1093/cercor/bhv151. Epub 2015 Jul 24. Cereb Cortex. 2016. PMID: 26209849 Free PMC article. - Pathological TDP-43 changes in Betz cells differ from those in bulbar and spinal α-motoneurons in sporadic amyotrophic lateral sclerosis.
Braak H, Ludolph AC, Neumann M, Ravits J, Del Tredici K. Braak H, et al. Acta Neuropathol. 2017 Jan;133(1):79-90. doi: 10.1007/s00401-016-1633-2. Epub 2016 Oct 18. Acta Neuropathol. 2017. PMID: 27757524 Free PMC article. - Neural Activity during Voluntary Movements in Each Body Representation of the Intracortical Microstimulation-Derived Map in the Macaque Motor Cortex.
Higo N, Kunori N, Murata Y. Higo N, et al. PLoS One. 2016 Aug 5;11(8):e0160720. doi: 10.1371/journal.pone.0160720. eCollection 2016. PLoS One. 2016. PMID: 27494282 Free PMC article. - Voluntary and reactive recruitment of locomotor muscle synergies during perturbed walking.
Chvatal SA, Ting LH. Chvatal SA, et al. J Neurosci. 2012 Aug 29;32(35):12237-50. doi: 10.1523/JNEUROSCI.6344-11.2012. J Neurosci. 2012. PMID: 22933805 Free PMC article. - From hand to mouth: monkeys require greater effort in motor preparation for voluntary control of vocalization than for manual actions.
Koda H, Kunieda T, Nishimura T. Koda H, et al. R Soc Open Sci. 2018 Nov 28;5(11):180879. doi: 10.1098/rsos.180879. eCollection 2018 Nov. R Soc Open Sci. 2018. PMID: 30564395 Free PMC article.
References
- Porter R, Lemon RN. Corticospinal Function and Voluntary Movement. Oxford: Clarendon; 1993. p. 428.
- Kwan HC, MacKay WA, Murphy JT, Wong YC. Spatial organization of precentral cortex in awake primates. II. Motor outputs. J Neurophysiol. 1978;41:1120–1131. - PubMed
- Lorente de Nò R. In: Physiology of the Nervous System. 3rd Ed. Fulton JF, editor. London: Oxford Univ Press; 1949. pp. 288–330.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources